Hydroxypropyl Betadex Analysis Service | Pharmaceutical Excipient
Hydroxypropyl betadex, a derivative of β-cyclodextrin, significantly improves its water solubility and inclusion ability by introducing hydroxypropyl substituents into the molecular structure. It can form stable inclusion complexes with a variety of drug molecules, thereby enhancing the solubility, stability, and bioavailability of drugs. Its analysis usually combines multiple advanced analytical methods to achieve a comprehensive evaluation. With low toxicity and excellent physicochemical properties, Hydroxypropyl Betadex Analysis Service is widely applied in drug development and quality control, providing key support for formulation design and excipient compliance research.
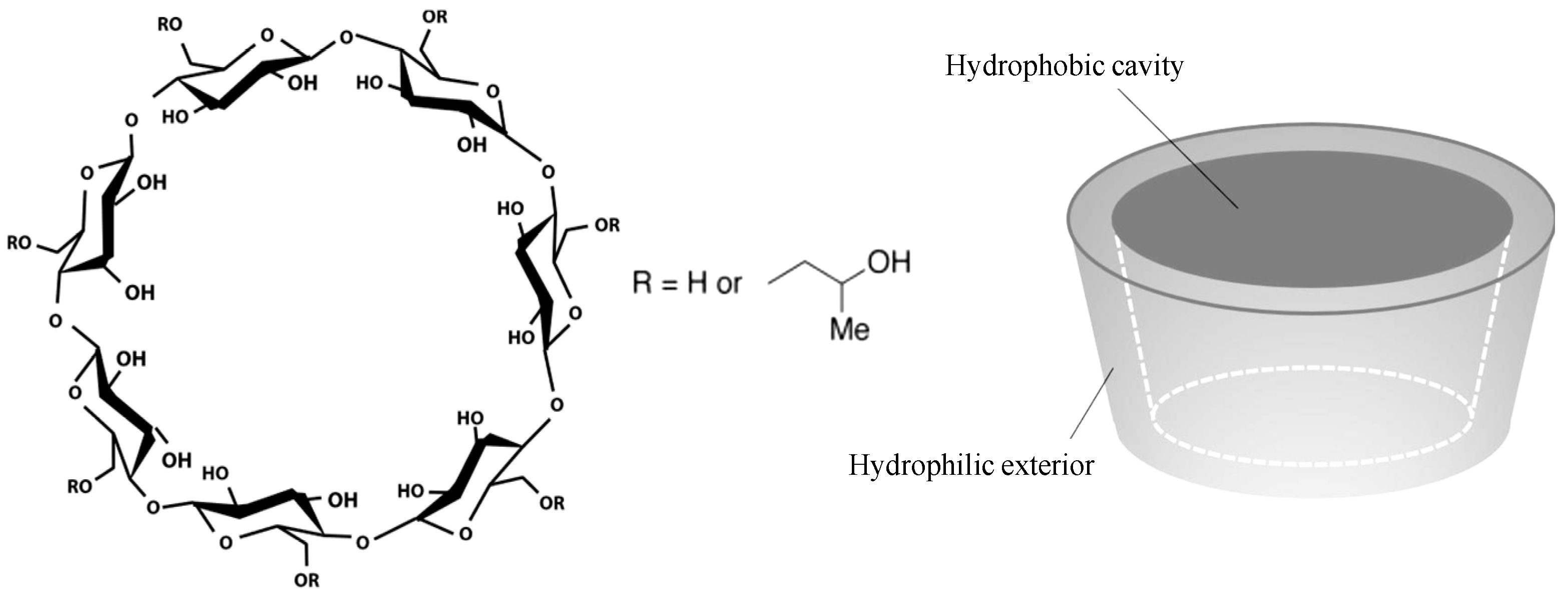
Stolzke, T. et al. Molecules, 2022.
Figure 1. Schematic Chemical Structure and Conformation of 2-Hydroxypropyl β-Cyclodextrin (HP-β-CD) .
Services at MtoZ Biolabs
Based on an advanced analysis platform, MtoZ Biolabs has launched the hydroxypropyl betadex analysis service based on pharmaceutical excipient to systematically evaluate the quality characteristics of hydroxypropyl betadex. The service relies on multiple analytical instruments to conduct comprehensive and precise analysis. The results include complete physicochemical parameter data and quantitative reports, providing reliable support for drug development, formulation design, and excipient compliance. The services provided by MtoZ Biolabs include but are not limited to the following:
1. Physical properties
Testing the basic characteristics of the sample, such as appearance and solubility, to ensure compliance with pharmaceutical excipient standards.
2. Content determination
Measuring the main component content through precise analytical methods to guarantee stable and controllable quality.
3. pH determination
Detecting the acidity or alkalinity of the solution to evaluate the suitability of the excipient in different formulation environments.
4. Chloride determination
Analyzing the chloride content in the sample to avoid potential impact on excipient stability and drug safety.
5. Conductivity determination
Assessing the ion concentration in the solution to reflect the total electrolytes, sample purity, and solution properties.
6. Related substances determination
Detecting impurity levels such as moisture and heavy metals to ensure the excipient meets safety and compliance requirements.
Sample Submission Suggestions
1. Sample type
Applicable to powdered or solid samples, which must ensure uniformity and representativeness to obtain stable and reliable test results.
2. Sample purity
It is recommended to remove impurities and moisture to avoid interference from foreign components in the detection of indicators such as content, pH, or chloride.
3. Sample storage
Samples should be stored under dry, light-protected, and appropriate temperature conditions to prevent changes in excipient properties caused by moisture or oxidation.
4. Sample transportation
During transportation, sealed containers should be used. If necessary, moisture-proof packaging or cold chain conditions should be applied to ensure the stability and integrity of the samples before reaching the testing platform.
Service Advantages
1. Comprehensive testing capability
By combining multiple analytical methods, the physicochemical properties and quality indicators of hydroxypropyl betadex can be systematically evaluated to ensure comprehensive and reliable results.
2. High accuracy and sensitivity
Relying on advanced analytical platforms, the content, pH, chloride, and impurity levels can be precisely measured to meet strict experimental requirements.
3. Professional technical team
Data interpretation and quality evaluation are provided by experienced experts, ensuring standardized processes and scientifically reliable results.
4. Efficient service process
Standardized and customized testing solutions are offered to shorten testing cycles and improve the efficiency of research and quality control.
Applications
1. Improvement of drug solubility
By detecting the inclusion ability of hydroxypropyl betadex, its application value in improving the solubility of poorly soluble drugs can be evaluated.
2. Formulation stability study
Hydroxypropyl betadex analysis service can be used to analyze its physicochemical properties under different environmental conditions to determine its impact on the stability of pharmaceutical formulations.
3. Optimization of drug delivery systems
Studying its binding performance with active ingredients to support oral, injectable, and inhalation delivery systems.
4. Biomedical material research
Hydroxypropyl betadex analysis service can be used to evaluate its compatibility and functional performance in bio-related materials to support the development of new excipients and carriers.
FAQ
Q1: Will moisture content or impurities affect the results?
A1: Yes. Moisture, heavy metals, and other impurities may interfere with the determination of pH, content, or conductivity. Therefore, it is recommended to properly preserve and prepare the samples before submission.
Q2: Can hydroxypropyl betadex be analyzed using UV?
A2: Conventional ultraviolet spectrophotometry is not suitable for directly detecting hydroxypropyl betadex. As a derivative of cyclodextrin, its main structure is a polysaccharide ring that lacks chromophores with conjugated double bonds or aromatic rings, resulting in almost no significant absorption in the ultraviolet region (200–400 nm).
Q3: What are the advantages of hydroxypropyl betadex compared with natural β-cyclodextrin?
A3: Compared with natural β-cyclodextrin, hydroxypropyl betadex has better water solubility and physicochemical stability, allowing for applications under a wider range of conditions. Its molecular structure modification provides greater flexibility and adaptability, resulting in superior performance in drug inclusion, solubility enhancement, and material property improvement.
How to order?







