Hydroxyethyl Cellulose Analysis Service | Pharmaceutical Excipient
Hydroxyethyl cellulose (HEC) is a non-ionic, water-soluble cellulose ether obtained by the controlled reaction of cellulose with ethylene oxide under alkaline conditions. This modification introduces hydroxyethyl groups into the cellulose backbone, improving solubility, viscosity control, and film-forming ability. HEC is widely used in pharmaceutical formulations as a binder, thickener, stabilizer, and controlled-release matrix. Its performance in dosage forms depends largely on molecular substitution patterns, viscosity grade, particle size, and purity.
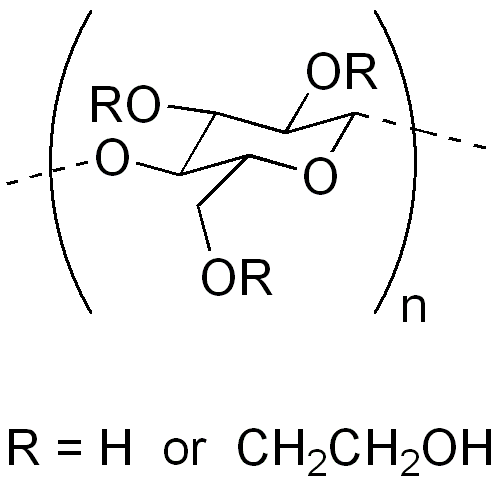
Figure 1. The Structure of Hydroxyethyl Cellulose
As the demand for consistent, high-quality pharmaceutical excipients continues to grow, rigorous testing and characterization of hydroxyethyl cellulose have become essential. Variability in degree of substitution, residual impurities, or microbial quality can significantly impact product performance and compliance with pharmacopeial standards. MtoZ Biolabs provides a systematic hydroxyethyl cellulose analysis service covering a comprehensive evaluation of its chemical, physical, and microbiological attributes to support pharmaceutical companies, research institutes, and quality control laboratories in ensuring safety, efficacy, and regulatory compliance.
Services at MtoZ Biolabs
HEC analysis focuses on both structural characteristics and functional properties. Key attributes include substitution degree, viscosity, moisture content, particle size distribution, pH, heavy metals, and microbial quality. MtoZ Biolabs applies validated analytical techniques to assess these parameters in line with pharmacopeial requirements. Our hydroxyethyl cellulose analysis service includes:
1. Appearance and Solubility Testing: Evaluation of powder color, clarity of solution, and dispersibility in water.
2. Identification: FTIR spectroscopy, viscosity profiling, and specific solubility behavior of hydroxyethylated cellulose.
3. Physicochemical Characterization: Determination of pH, loss on drying, ash content, viscosity grades, and degree of substitution.
4. Impurity and Residue Testing: Assessment of heavy metals, residual ethylene oxide or chlorides, and organic-soluble substances.
5. Particle Size Distribution and Flowability: Critical for consistency in blending and dosage form manufacturing.
6. Microbiological Testing: Enumeration of aerobic bacteria, yeasts, and molds, with absence tests for specified pathogens.
Analysis Workflow
1. Sample Receipt and Documentation: Verification of batch information and testing requirements.
2. Sample Preparation: Dispersion and dissolution under controlled conditions.
3. Physicochemical Testing: Execution of validated analytical methods for substitution degree, viscosity, and purity.
4. Microbiological Testing: Conducted under sterile laboratory conditions.
5. Data Processing and Comparison: Results benchmarked against pharmacopeial specifications or client standards.
6. Reporting: Delivery of a comprehensive report with compliance assessment and recommendations.
Service Advantages
1. Targeted Testing for Hydroxyethyl Cellulose
HEC requires evaluation of the substitution degree and viscosity, both critical to performance. MtoZ Biolabs provides tailored methods to ensure accurate profiling of these parameters.
2. Pharmacopoeia-Based and Validated Methods
All analyses are conducted according to USP, EP, and other regulatory standards. This guarantees results suitable for global regulatory submissions and quality audits.
3. Advanced Analytical Infrastructure
With state-of-the-art spectroscopic, chromatographic, and rheological instruments, combined with specialized expertise in cellulose derivatives, we provide reliable and reproducible results.
4. Comprehensive Reporting
Reports include methods, raw data, spectra, and compliance assessments, ensuring transparency and usability for regulatory or internal review.
5. Efficient Turnaround and Support
Standardized workflows shorten lead times. Dedicated technical support is available to help interpret results and advise on formulation implications.
Applications
1. Pharmaceutical Companies: For excipient qualification, new product development, and regulatory submissions.
2. Quality Control Departments: For raw material release testing and ongoing supplier qualification.
3. Research Institutions: For exploring new uses of hydroxyethyl cellulose in advanced dosage forms.
4. Global Registration: Supporting USP/EP-compliant data for international regulatory filings.
FAQs
Q1: How long does the hydroxyethyl cellulose analysis service take?
A1: Most projects are completed within 1–2 weeks, depending on scope.
Sample Submission Suggestions
1. Sample Type: Powdered hydroxyethyl cellulose.
2. Recommended Quantity: 50–100 g per batch for complete analysis.
3. Packaging: Sealed containers to prevent moisture absorption.
4. Transport: Room temperature transport; avoid prolonged exposure to high humidity.
5. Documentation: Include batch number, grade information, and specific testing requirements.
Deliverables
1. Sample information confirmation
2. Detailed methods and test procedures
3. Raw data and analytical spectra (e.g., FTIR, viscosity profiles)
4. Comprehensive statistical and numerical results
5. Compliance evaluation and conclusion
6. Risk notes and recommendations where applicable
Hydroxyethyl cellulose is a versatile pharmaceutical excipient whose performance depends on precise chemical and physical characteristics. Consistent quality and compliance are vital for ensuring drug safety and efficacy. MtoZ Biolabs provides a reliable hydroxyethyl cellulose analysis service, integrating advanced technology and regulatory expertise to support excipient quality control and regulatory compliance.
If you require professional and efficient testing for hydroxyethyl cellulose, contact MtoZ Biolabs today. We are committed to delivering accurate results and helping you achieve confidence in your excipient quality.
How to order?







