Hyaluronan Analysis Service
Hyaluronan Analysis Service is a comprehensive evaluation service for hyaluronic acid, assessing its chemical structure, purity, molecular weight distribution, physicochemical properties, and biocompatibility. This service provides scientific data and support for drug development, excipient quality control, and biomaterials research.
Hyaluronan (HA) is a naturally occurring high-molecular-weight polysaccharide widely used in pharmaceutical formulations, serving as a drug carrier, moisturizer, lubricant, and thickening agent. It plays a critical role in improving formulation stability, enhancing texture, and increasing drug bioavailability, while also showing potential applications in tissue repair and regenerative medicine.
Šimek M. et al. Carbohydr Polym. 2020.
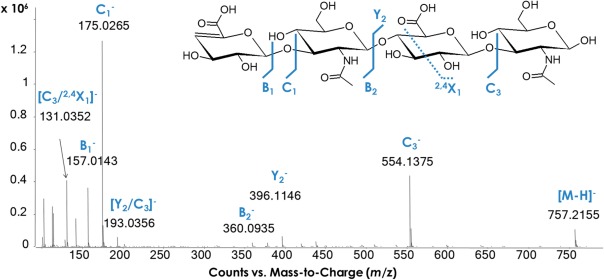
Figure 1. Negative ion ESI-MS fragmentation spectrum of unsaturated HA tetrasaccharide
Services at MtoZ Biolabs
MtoZ Biolabs offers a comprehensive Hyaluronan Analysis Service covering chemical structure, physicochemical properties, microbial safety, and biocompatibility. This service helps clients ensure the functionality, safety, and stability of hyaluronic acid in formulations, providing critical guidance for drug development and formulation optimization.
1. Chemical Composition and Structural Analysis
Advanced techniques, including mass spectrometry (MS), nuclear magnetic resonance (NMR), and Fourier-transform infrared spectroscopy (FTIR), are employed to characterize the molecular structure, chemical composition, and purity of hyaluronic acid.
2. Physical Property Analysis
Viscometers and rheometers are used to measure the viscosity, rheological behavior, solubility, and gelation ability of hyaluronic acid solutions and gels, providing essential data for evaluating formulation performance.
3. Thermal Property Analysis
Differential scanning calorimetry (DSC) and related methods are applied to assess melting point, enthalpy changes, and phase transition characteristics, offering insights for formulation process optimization.
4. Stability Analysis
The effects of temperature, humidity, light, and other environmental factors on hyaluronic acid stability are evaluated to guide proper storage and handling.
5. Microbial Testing
Total microbial count, mold, and yeast analyses are conducted to ensure the microbial safety of hyaluronic acid products.
6. Biocompatibility Assessment
In vitro cell assays and immunological analyses are performed to evaluate the compatibility of hyaluronic acid with biological tissues and cells, providing scientific support for its use as a drug carrier or in tissue engineering applications.
Service Advantages
1. Multidimensional Analytical Techniques
The service integrates analyses of chemical structure, physical properties, thermal behavior, microbial safety, and biocompatibility to provide comprehensive information.
2. Advanced Analysis Platform
MtoZ Biolabs established an advanced Hyaluronan Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
3. Customized Services
Analytical methods and parameters can be flexibly selected based on specific client requirements.
4. Cross-Disciplinary Applicability
Suitable for a variety of sample types across pharmaceuticals, regenerative medicine, biomaterials, and cosmetic applications.
Sample Submission Suggestions
1. Sample Types
Various types of hyaluronan samples are accepted, including purified powders or lyophilized samples, aqueous or buffer-dissolved solutions, hyaluronan-containing drug formulations, or composite materials.
2. Storage and Transport
Samples are recommended to be stored in a sealed, light-protected, and low-temperature environment. Powder samples can be transported at room temperature, while solutions are recommended to be shipped on dry ice.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Hyaluronan Analysis Service has broad application value in the pharmaceutical and related fields:
1. Pharmaceutical Formulation Development
By assessing molecular weight distribution, purity, and stability, it ensures the safety and effectiveness of hyaluronan as an excipient in injectables, sustained-release systems, and topical formulations.
2. Tissue Engineering and Regenerative Medicine
By evaluating viscoelasticity, solubility, and biocompatibility, it provides scientific support for applications in cartilage repair, wound healing, and cell carrier systems.
3. Biomaterials and Cosmetics
By analyzing the structural characteristics and moisturizing performance of hyaluronan, it supports the development of skin care, anti-aging, and advanced medical aesthetic products.
How to order?







