HPLC Peptide Purity Analysis Service
High performance liquid chromatography (HPLC) is a modern chromatographic separation technique extensively utilized in biological, chemical, and medical research. For peptide purity analysis, HPLC identifies the composition and content of peptides in samples, assessing the purity and impurities. Peptides are dissolved in a mobile phase and separated using a chromatographic column containing a stationary phase. Different components in the peptide sample exhibit distinct retention times based on their interactions. The detector analyzes retention time and signal intensity to qualitatively and quantitatively assess peptide purity.
Analysis Workflow
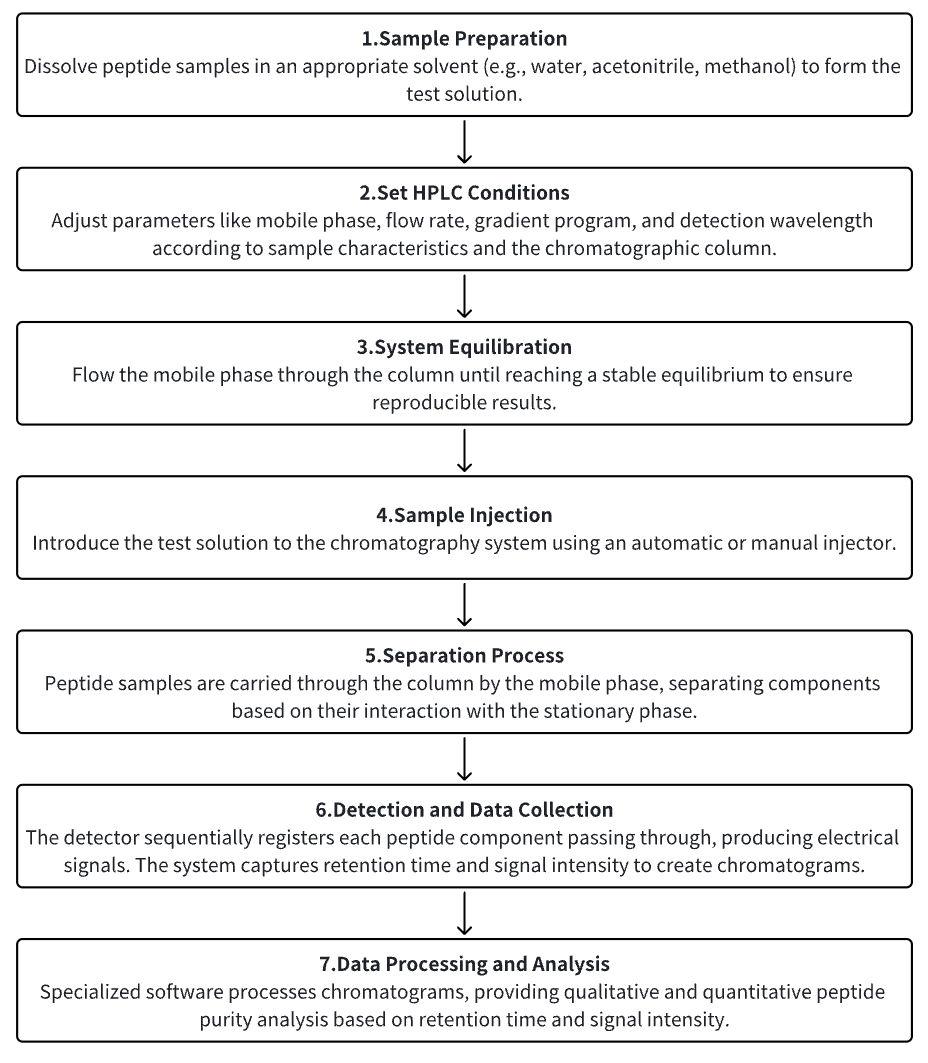
1. Sample Preparation
Dissolve peptide samples in an appropriate solvent (e.g., water, acetonitrile, methanol) to form the test solution.
2. Set HPLC Conditions
3. System Equilibration
4. Sample Injection
5. Separation Process
6. Detection and Data Collection
7. Data Processing and Analysis
Applications
1. Drug Development and Preparation
Assess peptide drug purity, composition, impurities, and concentration to ensure drug quality and safety while enabling high-purity peptide preparation.
2. Industrial Production
Monitor purification steps, degradation, and impurity production during peptide manufacturing to guide process optimization and quality control.
3. Clinical Diagnosis
Measure peptide levels in biological samples (e.g., serum, urine) to support clinical diagnosis, disease monitoring, and treatment evaluation.
4. Food Safety
Confirm peptide additive and residue purity in food, ensuring safety and quality.
5. Environmental Monitoring
Detect peptide pollutants in environmental samples (e.g., water, soil) to aid environmental risk assessment and pollution control.
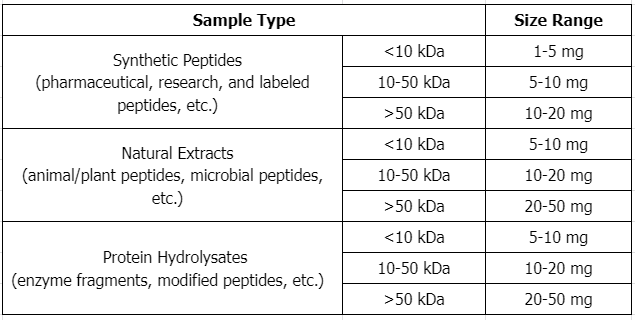
Sample Submission Requirements
Deliverables
In the technical report, MtoZ Biolabs will provide you with a detailed technical information, including:
1. Experimental Procedures
2. Relevant Chromatographic Parameters
3. HPLC Peptide Purity Analysis Detailed Information
4. Chromatography Images
5. Raw Data
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
* For research use only. Not for diagnostic purposes. Samples from individual customers or for personal use are not accepted.
Related Services
How to order?







