How to Reduce High Background Signals in Co‑IP Assays?
- Nonspecific binding: for example, unintended interactions between unrelated proteins and antibodies, Protein A/G beads, vectors, or epitope tags.
- Contamination derived from the antibody itself: particularly problematic when heavy-chain detection interferes with downstream analyses.
- IgG heavy-chain interference: during Western blotting, the ~50-kDa IgG heavy chain often migrates close to the target protein, potentially causing misinterpretation.
- Non-physiological interactions induced by excessive input protein or overexpression systems.
- Antibodies with insufficient affinity tend to generate nonspecific interactions.
- Unpurified antibodies (e.g., polyclonal sera or whole serum) contain substantial impurities.
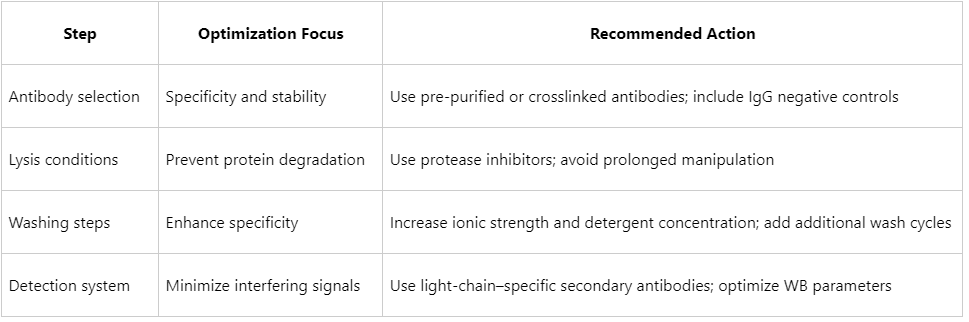
- Prefer validated monoclonal antibodies or pre-purified antibody preparations.
- Crosslink antibodies to magnetic beads (e.g., Protein A/G beads) to prevent heavy-chain leakage into Western blot analyses.
- Include an IgG negative control to assess binding specificity.
- Washing buffers with low ionic strength or insufficient wash cycles.
- Weak nonspecific interactions may persist under insufficient washing.
- Increase NaCl concentration to 300–500 mM.
- Add 0.1–0.5% NP-40 or Triton X-100.
- Extend washing duration or increase the number of washes (e.g., 5–6 cycles, 5 minutes each).
- When applying high-salt or relatively elevated washing temperatures (up to ~10°C), ensure that the stability of the target protein is maintained.
- Use lysis buffers suitable for the stability of interacting proteins, such as RIPA or NP-40–based buffers.
- Supplement with protease and phosphatase inhibitors.
- Limit lysis on ice to within 30 minutes.
- Whenever possible, validate interactions under endogenous expression conditions.
- For overexpression systems, reduce transfection dosage or use stable-expression models.
- Include empty-vector controls or validate by swapping different tags.
- Secondary antibodies raised in the same species as the IP antibody may detect IgG heavy chains.
- Insufficient membrane blocking or overly concentrated antibody incubation may generate background bands.
- Use light-chain-specific secondary antibodies (LCS).
- Employ HRP-conjugated tag antibodies (e.g., anti-HA-HRP) to minimize secondary-antibody cross-reactivity.
- Utilize signal-enhancing reagents to improve Western blot performance.
- Strengthen membrane blocking, such as by using 5% BSA instead of milk.
- Misconception 1: Excessive washing will remove all proteins.
- Misconception 2: More tags facilitate Co-IP.
- Recommendation: Incorporating mass-spectrometry analysis (IP-MS) after Co-IP provides a more comprehensive profile of interacting proteins and prevents over-reliance on Western blot signals.
High background signals frequently occur in Co-immunoprecipitation (Co-IP) experiments and often present as nonspecific bands, substantial interference in Western blotting, or poorly resolved target protein signals. These issues compromise data reliability and may obscure genuine protein–protein interaction events. To improve assay specificity and reproducibility, this article provides a systematic analysis of potential causes and corresponding optimization strategies across four dimensions: experimental design, reagent selection, workflow, and data interpretation.
What Constitutes Co-IP Background Signal?
Co-IP background signals primarily arise from the following sources:
Common Causes of High Co-IP Background and Recommended Optimization Strategies
1. Antibody Selection and Quality Control
(1) Causes
(2) Optimization strategies
2. Inadequate or Overly Mild Washing Conditions
(1) Causes
(2) Optimization strategies
3. Suboptimal Composition of the Lysis Buffer
(1) Cause
Overly mild buffers or the absence of protease/phosphatase inhibitors can lead to protein degradation or increased nonspecific binding.
(2) Optimization strategies
4. Excessive Input Protein or Overexpression-Induced Artifacts
(1) Causes
High protein abundance or tag-fusion overexpression may induce non-physiological associations or artificial collision artifacts.
(2) Optimization strategies
5. Inappropriate Western Blot Detection Conditions
(1) Causes
(2) Optimization strategies
Recommended Systematic Workflow for Reducing Co-IP Background Signals
Common Misconceptions and Practical Recommendations
Reality: Stable interacting proteins generally withstand stringent washing conditions.
Reality: Multiple tags may promote nonspecific interactions or aggregation.
Although high background signals in Co-IP assays are common, they are fully addressable. Refining experimental design, selecting appropriate reagents, and optimizing the workflow can substantially improve data clarity and reproducibility. For researchers facing challenges in Co-IP or subsequent mass-spectrometry workflows, MtoZ Biolabs offers integrated solutions supported by high-affinity antibody libraries, protein-purification platforms, and high-resolution Orbitrap mass spectrometry. Our team has extensive hands-on experience in optimizing washing conditions and reducing background interference, enabling the acquisition of clearer and more reliable protein-interaction data.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







