How to Extract Membrane Proteins Using CNBr Cleavage Protocols?
- The reaction must be performed under anhydrous acidic conditions, typically in 70% formic acid or 6 M HCl.
- Reactions should be carried out in the dark to prevent CNBr decomposition.
- Prior reduction and alkylation of disulfide bonds are required to enhance cleavage efficiency.
- CNBr should not be used for peptides terminating with Met at the C-terminus, as cyclic by-products may form.
- CNBr is highly toxic and must be handled in a fume hood.
- After the reaction, formic acid should be removed by SpeedVac drying or lyophilization.
- Specialized membrane protein extraction and CNBr cleavage-based mass spectrometry services
- Standardized sample processing workflows ensuring reproducibility and comparability
- High-resolution mass spectrometry platforms (Orbitrap Exploris, Q Exactive, etc.)
- AI-assisted database searching and protein annotation for accurate membrane protein identification
Membrane proteins, characterized by strong hydrophobicity, complex three-dimensional conformations, and low abundance within cellular membranes, present substantial challenges in proteomic investigations. Conventional trypsin digestion often exhibits poor efficiency in membrane protein samples, resulting in low identification rates and poor reproducibility. In contrast, the cyanogen bromide (CNBr) cleavage method, owing to its specific recognition of methionine (Met) residues and cleavage at their C-termini, has emerged as a powerful alternative for membrane protein analysis. This article provides a systematic overview of CNBr cleavage-based membrane protein extraction, including its underlying principles, experimental workflow, critical precautions, and technical advantages. Furthermore, in conjunction with the MtoZ Biolabs platform, we discuss strategies to enhance both the depth and reliability of membrane protein mass spectrometry studies.
Principle of CNBr Cleavage
CNBr is a chemical reagent that specifically cleaves peptide bonds at the C-terminal side of methionine residues, yielding peptides with uniform termini, homoserine lactone at the C-terminus, and a free amino group at the N-terminus.
Reaction Conditions for CNBr Cleavage:
Experimental Workflow for CNBr-Based Membrane Protein Extraction
1. Membrane Protein Enrichment
Typical approaches include:
(1) Differential centrifugation to isolate membrane fractions
(2) Ultracentrifugation (e.g., 100,000 × g) to remove cytosolic proteins and retain membrane proteins
(3) Use of nonionic or zwitterionic detergents (e.g., Triton X-100, CHAPS) to solubilize hydrophobic proteins
2. Protein Denaturation, Reduction, and Alkylation
(1) Denature proteins completely using 6 M guanidine hydrochloride or 8 M urea
(2) Reduce disulfide bonds with DTT or TCEP
(3) Alkylate free thiols with iodoacetamide (IAA) to prevent reformation of disulfide linkages
3. CNBr Cleavage Reaction
(1) Dilute the protein solution to 70% formic acid
(2) Add solid CNBr powder at a 3–5-fold molar excess relative to methionine residues
(3) Incubate at room temperature for 12–24 hours in the dark
Precautions:
4. Peptide Purification and Mass Spectrometry Analysis
(1) Purify and concentrate peptides using solid-phase extraction (SPE)
(2) Analyze peptides via LC–MS/MS platforms such as Orbitrap or Q-TOF
(3) Perform database searches with parameters accommodating non-tryptic cleavage, specifying CNBr cleavage as the enzyme rule
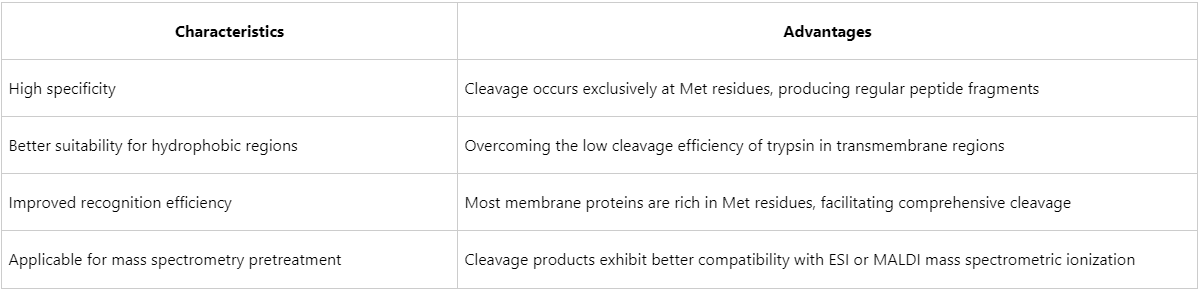
Advantages of CNBr Cleavage in Membrane Proteomics
Representative Applications
1. Structural Studies of Transmembrane Receptors (e.g., GPCRs)
CNBr cleavage generates domain-specific peptides that facilitate post-translational modification (PTM) identification and structural conformational analysis.
2. Quantitative Membranome Analysis
When combined with labeling strategies such as TMT or iTRAQ, CNBr treatment improves the coverage and reliability of quantitative membrane proteomics.
3. Biomarker Discovery in Disease-Associated Membrane Proteins
Many critical biomarkers within tumor microenvironments, such as EGFR and PD-L1, are membrane-localized proteins. CNBr cleavage enhances their targeted detection and validation.
Advantages and Service Capabilities of MtoZ Biolabs
MtoZ Biolabs offers:
The CNBr cleavage-based membrane protein extraction approach, featuring high specificity and broad applicability, is increasingly recognized as a key strategy in membrane proteomics. Particularly in high-throughput, quantitative, or post-translational modification studies, its utility continues to grow. Researchers engaged in membrane protein projects are encouraged to contact MtoZ Biolabs for comprehensive, customized solutions aimed at maximizing proteomic insights and advancing scientific discovery.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







