Guar Gum Analysis Service | Pharmaceutical Excipient
The Guar Gum Analysis Service focuses on systematic testing and comprehensive quality evaluation of guar gum as a pharmaceutical excipient. Guar gum is a natural polysaccharide extracted from guar seeds, primarily composed of galactomannan. Its molecular backbone consists of D-mannopyranose units linked via β-1,4 bonds, with branches of D-galactopyranose connected by α-1,6 bonds, maintaining an approximate mannose-to-galactose ratio of 2:1. This unique structure imparts strong hydrophilicity, thickening ability, and film-forming capacity, making guar gum widely applied in pharmaceutical formulations as a binder, disintegrant, suspension stabilizer, and controlled-release matrix. In addition, guar gum also serves as a thickener in the food and cosmetics industries, and as an eco-friendly additive in papermaking.
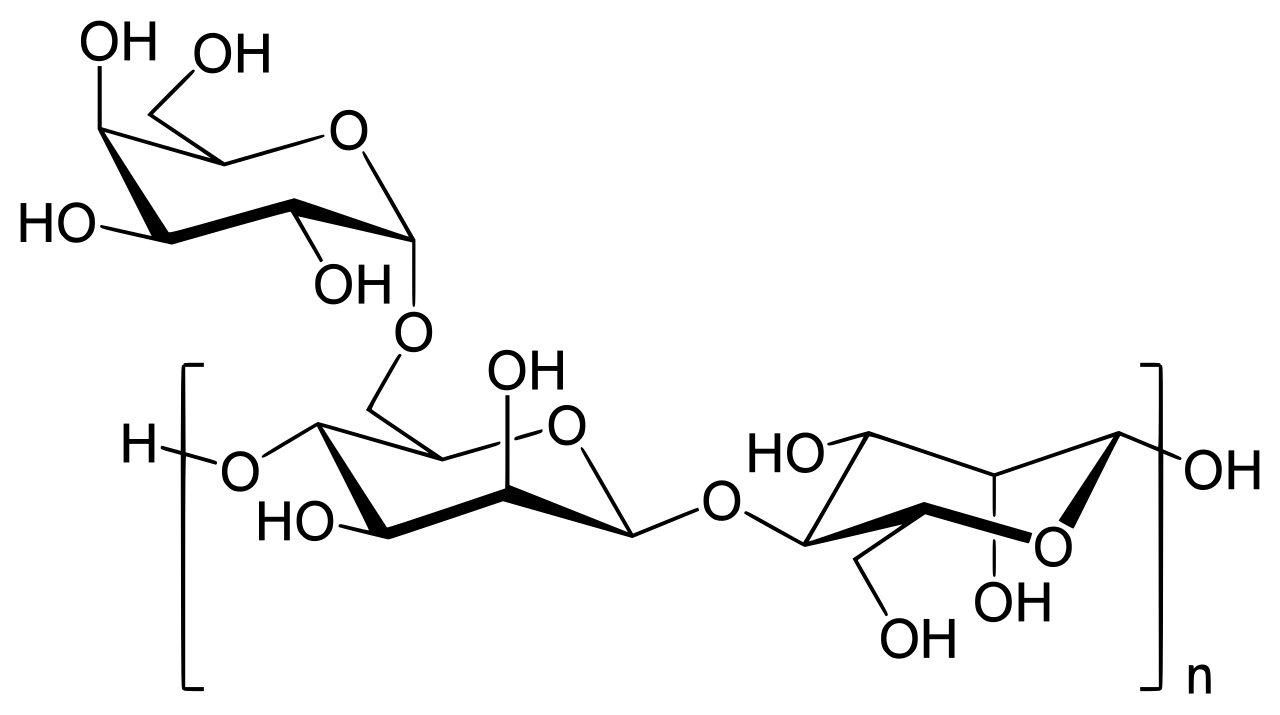
Figure 1. Molecular Structure of Guar Gum
Leveraging an advanced quality control platform and an interdisciplinary team, MtoZ Biolabs provides Guar Gum Analysis Service to deliver systematic physicochemical characterization, impurity control, and safety assessment, ensuring the stability and compliance of excipients in formulation development and industrial production.
Services at MtoZ Biolabs
MtoZ Biolabs' Guar Gum Analysis Service covers physicochemical properties, impurity profiling, and functional performance testing, including:
● Microscopic Identification: Examination of microscopic morphology and structural features using microscopy and iodine staining to confirm guar gum identity.
● Content Determination: Quantitative assessment of guar gum by high-performance liquid chromatography (HPLC).
● Impurity Profiling: Analysis of common impurities such as starch (iodine reaction), protein (nitrogen determination), and borates (colorimetric methods) to evaluate purity and quality.
● Physicochemical Parameters: Measurement of solubility, viscosity, pH, acid-insoluble matter, and moisture loss on drying.
● Microbial Limits: Determination of bacterial and fungal contamination using plate count, membrane filtration, and most probable number (MPN) methods.
● Functional Performance Evaluation: Testing of gelation, thickening, and controlled-release properties to support formulation development and process optimization.
Why Choose MtoZ Biolabs?
✅ Multi-Technology Platform Support: Integration of HPLC, ICP-MS, rheological analysis, and microbiological testing to comprehensively cover key quality attributes of guar gum.
✅ Comprehensive Testing Scope: Assessment of structural and functional characteristics alongside impurity and safety evaluations, generating results with higher reference value.
✅ Flexible Testing Solutions: Customized testing packages designed according to R&D stage or regulatory submission requirements.
✅ Expert Team Support: Experienced scientists with extensive knowledge of pharmacopeial standards and industry regulations ensure scientific reliability of the results.
✅ Transparent Reporting: Delivery of experimental data, chromatograms, and integrated evaluations that can be directly applied to research and quality management.
Sample Submission Suggestions
To ensure reliable and consistent testing results, we recommend the following for sample submission:
Sample Type: Powdered guar gum, minimum 10 g.
Storage Conditions: Store at room temperature in a dry, light-protected environment to prevent moisture absorption.
Transport: Use sealed packaging to avoid contamination and water uptake.
Additional Information: Provide sample source, batch number, and testing objectives to facilitate the design of an appropriate analytical plan.
Guar Gum Analysis Service is of great importance in pharmaceutical formulation development and quality control, optimization of food industry formulations, and research in papermaking and materials science. Comprehensive physicochemical and safety testing enables clients to accurately understand the key characteristics of guar gum and to ensure excipient stability and compliance across diverse applications.
MtoZ Biolabs remains dedicated to providing high-standard excipient analysis solutions and has accumulated extensive experience in the analysis of both natural and modified polysaccharides, including methylcellulose, hydroxypropyl cellulose, and xanthan gum. Contact us to obtain reliable data support and accelerate your R&D and quality management processes.
How to order?







