Gellan Analysis Service | Pharmaceutical Excipient
- Sample Types: Powder, solution, or gel samples are acceptable. For solutions, please specify the solvent type and concentration.
- Storage and Transportation: Samples should be stored sealed in a dry and light-protected environment. Cold chain or dry ice is recommended for long-distance transportation.
- Pharmaceutical Formulation Optimization: Use rheological and gel performance data to guide the design of sustained-release, controlled-release, and mucoadhesive formulations.
- Quality Control: Ensure batch-to-batch consistency and stability of gellan gum excipients.
- Drug Delivery System Development: Evaluate its suitability as a drug carrier, gel matrix, or film-forming material.
- Tissue Engineering and Biomaterials: Investigate its potential in scaffold fabrication, cell encapsulation, and biocompatibility applications.
Gellan Analysis Service is designed to comprehensively reveal the composition, molecular structure, and performance characteristics of gellan through systematic physicochemical testing, structural characterization, thermal, and rheological analysis. The service provides scientific support for formulation development, optimization of drug delivery systems, and quality control, and it also promotes the innovative application of pharmaceutical excipients in the pharmaceutical and biomaterials fields.
Gellan is an anionic polysaccharide produced by microbial fermentation and is mainly composed of glucose, galactose, and glucuronic acid. With excellent gelling ability, film-forming property, rheological characteristics, and biocompatibility, gellan is widely used as a pharmaceutical excipient in sustained-release formulations, oral liquids, suspensions, and tissue engineering scaffolds. Systematic structural and functional analysis of gellan not only helps to elucidate its mechanism of action in pharmaceutical formulations but also provides scientific evidence for formulation optimization, quality control, and the development of novel drug delivery systems.
Services at MtoZ Biolabs
MtoZ Biolabs provides a systematic Gellan Analysis Service that covers multiple dimensions of testing, including physicochemical properties, rheological behavior, thermal characteristics, and molecular structure.
1. Rheological Property Evaluation
Rheometers are used to measure the viscosity of gellan solutions and the relationship between shear stress and shear rate, thereby analyzing their flow behavior under different conditions and their role in drug release mechanisms.
2. Gel Performance Testing
The viscosity, elasticity, and mechanical properties of gellan gum are evaluated to determine their impact on drug encapsulation efficiency and stability.
3. Thermal Property Analysis
The melting point, enthalpy of phase transition, and thermal stability of gellan are investigated to reveal performance changes during processing and storage.
4. Composition and Structural Characterization
High-performance liquid chromatography (HPLC) is applied to quantify gellan content, while Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) are used to elucidate its molecular structure and functional group features.
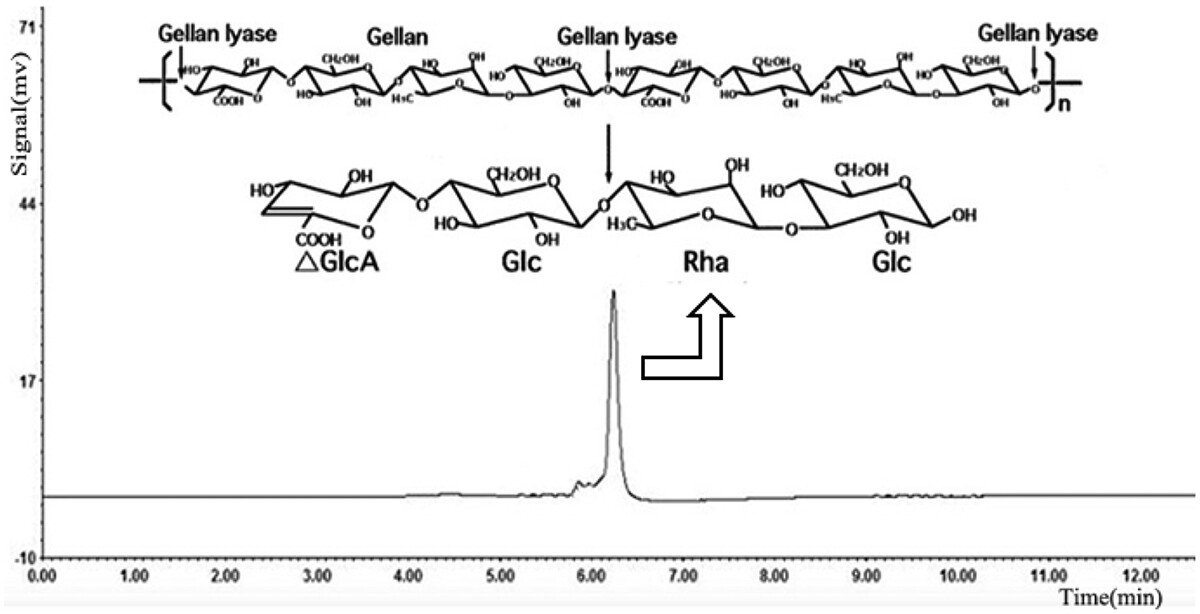
Li A. et al. Bioengineered. 2019.
Figure 1. HPLC Analysis of the Gellan Degradation Products
Service Advantages
1. Advanced Analysis Platform
MtoZ Biolabs established an advanced Gellan Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. Comprehensive Multidimensional Testing
Integrates physicochemical, thermal, structural, and rheological analyses to characterize gellan gum from multiple perspectives.
3. Customized Solutions
Testing items can be flexibly combined according to the client’s research objectives, providing tailored analytical strategies.
4. Experienced Scientific Team
A team of experts with years of experience in pharmaceutical excipients and formulation development delivers professional data interpretation and application guidance.
Sample Submission Suggestions
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
How to order?







