Galactomannan Analysis Service
Galactomannan is a type of natural polysaccharide composed of mannose and galactose units linked by glycosidic bonds. It is widely found in the seeds of leguminous plants (such as guar and locust bean), yeast cell walls, and certain microbial sources. Its structure typically consists of a mannose backbone with galactose side chains, exhibiting excellent hydrophilicity and rheological properties. Due to its high viscosity, solubility, and biocompatibility, galactomannan is extensively used as a thickener, stabilizer, and emulsifier in the food industry, as well as in pharmaceutical and biomaterial fields for drug delivery systems, tissue engineering scaffolds, and biocolloid development.
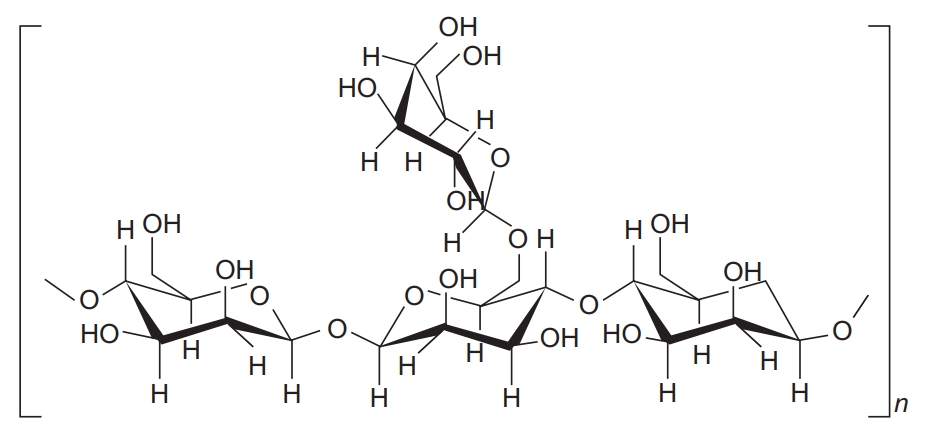
Eddouks, M. et al.Studies in Natural Products Chemistry, 2014.
Figure 1. Galactomannan
Services at MtoZ Biolabs
Based on advanced mass spectrometry and multi-dimensional characterization platforms, the galactomannan analysis service launched by MtoZ Biolabs provides systematic analysis of the composition, structural features, and physicochemical properties of galactomannan, helping researchers gain in-depth insight into its molecular structure and functional characteristics. The service includes, but is not limited to, the following aspects:
1. Monosaccharide Composition and Ratio Analysis
High-performance liquid chromatography (HPLC) and mass spectrometry (MS) are used to quantitatively determine the ratio of mannose to galactose, revealing the linkage characteristics between the backbone and side chains.
2. Structural and Conformational Analysis
Nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FTIR), and ultraviolet spectroscopy (UV) are applied to analyze the backbone structure, side-chain distribution, and glycosidic bond types, determining spatial conformation and branching features.
3. Molecular Weight and Degree of Polymerization Determination
Liquid chromatography–mass spectrometry (LC-MS/MS) or gel permeation chromatography (GPC) is used to determine molecular weight distribution and degree of polymerization, assessing the uniformity and structural complexity of the sample.
4. Morphology and Surface Structure Observation
Scanning electron microscopy (SEM) and cryo-electron microscopy (Cryo-EM) are combined to observe the microscopic morphology, aggregation state, and surface structural characteristics of galactomannan.
5. Thermal Properties and Stability Evaluation
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) are employed to study the thermal stability, decomposition temperature, and physical transition behavior of galactomannan.
6. Functional Group and Impurity Detection
Elemental analysis and high-resolution mass spectrometry (HR-MS) are used to detect functional groups such as sulfate and acetyl groups, as well as impurities, to assess sample purity and modification levels.
Sample Submission Suggestions
1. Sample Type
Purified galactomannan powders, lyophilized samples, or solutions are acceptable. Plant extracts or composite samples can also be analyzed. A sample purity of ≥90% is recommended to ensure the accuracy and reproducibility of analytical results.
2. Sample Storage
Solid samples should be sealed and stored in a dark environment at 4°C or −20°C. Solution samples should be refrigerated at low temperatures and protected from repeated freeze-thaw cycles to prevent structural degradation.
3. Sample Transportation
Sealed and moisture-proof packaging is recommended. Liquid samples should be transported under cold-chain conditions, while powder samples can be shipped at room temperature for short periods but should be kept away from heat, humidity, and direct light exposure.
Service Advantages
1. High-Resolution Analytical Capability
Equipped with advanced instruments such as LC-MS/MS, FTIR, NMR, and Cryo-EM, enabling precise characterization from the molecular level to microscopic morphology.
2. Extensive Polysaccharide Research Experience
The technical team has years of experience in plant and functional polysaccharide research, with deep knowledge of the structural characteristics of galactomannans from different sources.
3. Strict Quality Control System
Multiple quality control checkpoints are established throughout sample preparation and data reporting to ensure result stability and reproducibility.
4. Customized Analytical Solutions
Analytical combinations can be flexibly adjusted according to sample properties and research goals to meet the needs of food, pharmaceutical, and material-related studies.
Applications
1. Plant Seed Polysaccharide Research
The galactomannan analysis service can be used to characterize the structural composition and molecular features of naturally derived galactomannans from leguminous plants and other botanical sources.
2. Functional Activity Studies
By investigating the antioxidant, antimicrobial, and cell-interaction properties of galactomannan, the service supports its potential applications in functional foods and skincare formulations.
3. Storage and Processing Stability Evaluation
The galactomannan analysis service can assess structural variations and thermal stability of galactomannan under different conditions, providing scientific guidance for product storage and processing.
4. Pharmaceutical Excipient Quality Assessment
Through analysis of molecular distribution, viscosity, and compatibility of galactomannan in pharmaceutical formulations, the service ensures its stability and safety in excipient applications.
FAQ
Q1: Can the Branched Structure of Galactomannan Be Analyzed?
A1: Yes. By combining nuclear magnetic resonance (NMR) and Fourier-transform infrared spectroscopy (FTIR), branching linkage sites, substituent characteristics, and spatial conformations can be identified, allowing determination of branching density and stereochemical configuration.
Q2: Will Different Extraction Methods Affect the Analysis Results?
A2: Yes. Extraction conditions such as temperature, solvent, pH, and deproteinization procedures may alter the molecular weight, branching ratio, or acetylation level of galactomannan. Therefore, it is recommended to specify the extraction method or experimental requirements when submitting samples to ensure data accuracy.
Q3: Can Batch-to-Batch Consistency Analysis Be Performed?
A3: Yes. MtoZ Biolabs can conduct structural comparison and compositional analysis across different sample batches to evaluate the consistency of galactomannan in terms of molecular weight, composition, and physicochemical properties.
How to order?







