Formulation Development Service | Cryo-EM
Formulation development is a critical phase in pharmaceutical research and drug product optimization, directly influencing therapeutic efficacy, safety, stability, and manufacturability. As drug modalities become more complex—including biologics, nanoparticles, gene therapies, and combination products—the need for detailed structural characterization during formulation becomes increasingly essential. For nanoparticle-based formulations and gene therapy vectors in particular, attributes such as particle size, morphology, surface properties, encapsulation efficiency, and aggregation behavior have a profound impact on pharmacokinetics, biodistribution, therapeutic performance, and immunogenicity. Even minor structural variations can lead to significant differences in stability, bioavailability, and clinical outcomes, highlighting the importance of precise structural assessment in formulation design and process optimization. Cryogenic electron microscopy (Cryo-EM) has emerged as a powerful technology to meet this need, offering near-atomic resolution imaging under native-like conditions. By directly visualizing morphological features, size distributions, internal payload organization, and aggregation phenomena, Cryo-EM provides unparalleled insights into formulation structure-function relationships, enabling stable and effective formulation development.
Service at MtoZ Biolabs
MtoZ Biolabs provides a dedicated Formulation Development Service utilizing Cryo-EM, delivering direct, high-resolution structural insights to support formulation optimization across all stages of pharmaceutical development. By preserving pharmaceutical samples in a vitrified state, Cryo-EM enables artifact-free imaging of morphology, surface characteristics, and internal organization under native-like conditions. Within our Formulation Development Service, Cryo-EM enables:
✔️Particle Morphology Assessment: Evaluating vesicle, nanoparticle, or carrier shape and surface uniformity.
✔️Size Distribution Analysis: Measuring particle size variability to optimize homogeneity.
✔️Aggregation and Stability Profiling: Detecting early aggregation or degradation events under formulation or stress conditions.
✔️Encapsulation and Loading Visualization: Verifying payload incorporation efficiency and internal structure of delivery systems.
✔️Batch Consistency Evaluation: Monitoring consistency across formulation lots during optimization.
These capabilities directly support rational formulation design, process scalability, and quality attribute control.
Analysis Workflow
1. Consultation and Study Design
We collaborate with clients to define formulation objectives, characterization needs, and Cryo-EM analysis strategies.
2. Sample Preparation and Vitrification
Samples are prepared under optimized vitrification protocols to preserve structural integrit.
3. Cryo-EM Imaging and Data Acquisition
High-resolution imaging is performed under low-dose conditions to capture true-to-native morphology and internal structure.
4. Structural Analysis and Formulation Optimization Support
Analysis includes size distribution profiling, morphological assessment, aggregation evaluation, encapsulation visualization, batch consistency studies, and more.
5. Report Delivery
Clients receive detailed reports containing representative images, quantitative structural metrics, and interpretation to inform formulation adjustments and decision-making.
Service Advantages
✅Full Development Support: We cover structural evaluation needs from early screening to late-stage formulation validation.
✅Tailored Analysis Plans: We design flexible, project-specific analysis strategies based on formulation goals.
✅Fast and Consistent Delivery: We ensure rapid turnaround and reliable data output through optimized workflows and project coordination.
✅Comprehensive Reports: We deliver detailed structural analysis reports with clear, actionable formulation recommendations.
Applications
1. Screening of Early-Stage Formulations
We evaluate particle morphology, size distribution, and encapsulation efficiency to help identify promising initial formulations for further development.
2. Optimization of Formulation Parameters
Our Cryo-EM analysis provides detailed structural feedback on formulation adjustments, supporting refinement of composition, process conditions, and excipient selection.
3. Stability and Stress Testing Support
We monitor structural changes under accelerated or real-time storage conditions, helping assess the robustness of formulations during stability studies.
4. Batch Consistency Assessment
We verify the morphological and structural consistency of pilot-scale and production batches, supporting process control and scale-up validation.
5. Formulation Comparability Studies
Our service enables side-by-side structural comparisons of different formulation versions, aiding decision-making during formulation bridging or change management.
Case Study
Formulation Development of Butamben-Loaded Nanostructured Lipid Carriers
Local acidosis reduces the efficacy of conventional anesthetics. Although butamben (BTB) can overcome this limitation due to its non-ionizable structure, its poor solubility poses a challenge. To address this, a nanostructured lipid carrier (NLC) formulation encapsulating BTB was developed. The formulation was optimized through factorial design and characterized by DLS, XRD, DSC, and Cryo-EM. Cryo-EM analysis revealed uniform nanoparticle morphology, supporting high encapsulation efficiency (99.5%) and structural stability over 360 days at room temperature. The optimized NLCBTB formulation demonstrated prolonged drug release, reduced in vitro cytotoxicity, and improved analgesic effectiveness in an inflammatory hyperalgesia model. Cryo-EM provided critical morphological confirmation that contributed to validating the formulation's stability and performance.
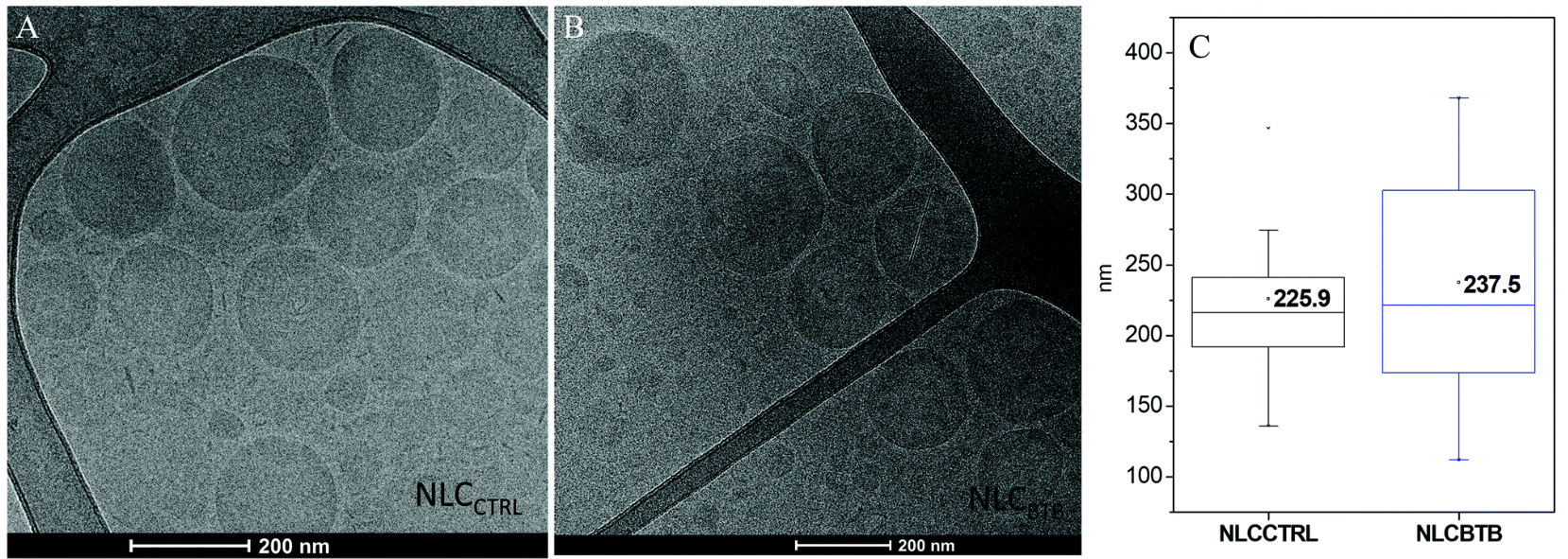
Figure 1. Cryo-EM Micrographs of the Nanostructured Lipid Carrier without (A) and with (B) Butamben.
(C) Average Size of NLC, Determined from the Cryo-EM Images, Using ImageJ Software.
MtoZ Biolabs empowers drug developers with Cryo-EM-based structural analysis solutions, accelerating the creation of stable, effective, and scalable drug products. To learn more about our Formulation Development Service utilizing Cryo-EM, or to discuss your formulation optimization needs, please contact us.
How to order?







