Ethylcellulose Analysis Service | Pharmaceutical Excipient
Ethylcellulose Analysis Service provides systematic evaluation of ethylcellulose, including structure, substitution degree, molecular weight distribution, purity, physicochemical properties, and application performance. These results support pharmaceutical development, excipient quality control, and functional material studies. MtoZ Biolabs ensures accuracy, reproducibility, and compliance through advanced platforms and an experienced team.
Ethylcellulose (EC) is a nonionic cellulose derivative modified by partial hydroxyethylation. With a 1,4-β-glucosidic backbone, it offers excellent film-forming ability, hydrophobicity, and thermal stability, along with non-toxicity and biocompatibility. In pharmaceuticals, EC is widely used for controlled-release coatings and matrix materials. It is also applied in food, coatings, and cosmetics due to its stability and versatility.
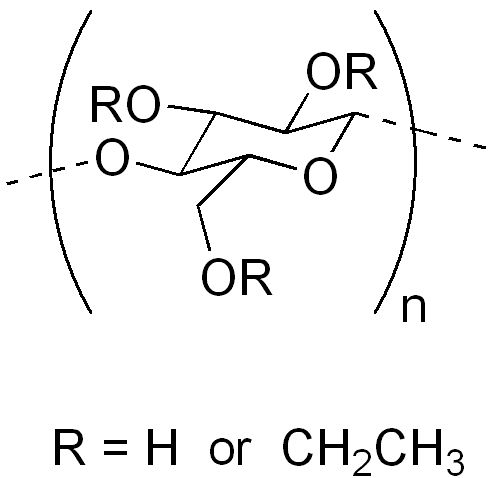
Figure 1. Molecular Structure of Ethylcellulose
Services at MtoZ Biolabs
MtoZ Biolabs provides a full suite of analytical solutions for EC, ranging from fundamental physicochemical characterization to advanced functional assessments. Our Ethylcellulose Analysis Service includes:
● Structure and Substitution Degree Analysis
Using nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FTIR), and elemental analysis, we accurately evaluate the degree and distribution of ethyl substitution, which reveals the essential structural features of EC.
● Molecular Weight and Distribution Measurement
Employing gel permeation chromatography (GPC), light scattering, and capillary viscometry, we determine molecular weight and its distribution, providing essential data for predicting material performance.
● Purity and Impurity Detection
High-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) are used to analyze residual solvents, byproducts, and potential impurities, thereby ensuring excipient safety and regulatory compliance.
● Physicochemical Property Testing
Solubility, viscosity, softening temperature, thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC) are included to provide a complete profile of processing behavior and stability.
● Application Performance Evaluation
For pharmaceutical applications, we test film-forming ability, moisture permeability, and release behavior. For other industries, we assess performance in coatings, barrier systems, and reinforcing materials.
Why Choose MtoZ Biolabs?
✅ Comprehensive Analytical Platforms: Equipped with NMR, FTIR, GPC, HPLC, GC-MS, TGA, and DSC, our laboratory covers every stage of characterization from structural analysis to impurity profiling and functional testing.
✅ Flexible Testing Schemes: Analytical strategies are tailored to customer needs, combining methods to address both basic quality control and advanced functional evaluations.
✅ Integrated Data Interpretation: Structural characteristics, physicochemical behavior, and application performance are analyzed together, enabling a deeper understanding of EC’s potential uses.
✅ Wide Sample Compatibility: Applicable to raw powders, pharmaceutical excipients, composite materials, and finished dosage forms, meeting diverse requirements from research to industrial production.
Sample Submission Suggestions
· Sample Types: Ethylcellulose powders, granules, films, or EC-containing formulations.
· Sample Amount: At least 1 g for routine testing; at least 100 mg for thermal and molecular weight analyses.
· Storage Conditions: Store at room temperature, protected from light and moisture; for impurity testing, samples should be sealed tightly.
· Transport Conditions: Room temperature transportation is recommended, but low temperature may be used for samples containing special components.
Note: Provide details on sample collection and handling. If you need further details, our technical support team is happy to assist and provide comprehensive guidance on sample submission.
Applications
Ethylcellulose Analysis Service has broad applications across multiple fields:
Pharmaceutical Research and Quality Control: Evaluating release characteristics, film-forming properties, and purity in controlled-release formulations.
Excipient Consistency Evaluation: Detecting structural and performance differences across batches to ensure manufacturing quality stability.
Materials Science Research: Characterizing thermal behavior and solubility to provide reference data for novel material development.
Food and Cosmetics Industries: Assessing the safety and functionality of EC when used in additives and cosmetic formulations.
What Could be Included in the Report?
1. Raw Data and Spectra
2. Substitution Degree and Molecular Weight Analysis
3. Purity and Impurity Reports
4. Thermal and Physicochemical Performance Data
5. Complete Experimental Report
FAQ
Q1: How long does testing take?
MtoZ Biolabs Ethylcellulose Analysis Service provides detailed and actionable results for pharmaceutical, material, food, and cosmetic applications. By combining advanced instrumentation with expert interpretation, we help clients evaluate excipient quality, support R&D, and accelerate industrial applications.
How to order?







