Drug Stability Testing Service | Cryo-EM
Ensuring the stability of pharmaceutical products throughout development, manufacturing, storage, and distribution is critical for maintaining therapeutic efficacy, safety, and regulatory compliance. Subtle structural changes at the nanoscale can significantly impact drug performance, particularly for complex modalities such as biologics, nanoparticle formulations, gene therapy vectors, and vaccine platforms. Cryogenic electron microscopy (Cryo-EM) offers a unique ability to directly visualize and monitor structural integrity at high resolution, capturing morphological changes. By preserving samples in a vitrified state, Cryo-EM enables true-to-native imaging, providing unparalleled insights into stability-related phenomena. MtoZ Biolabs offers a dedicated Drug Stability Testing Service using Cryo-EM, supporting pharmaceutical and biotechnology companies in conducting robust, data-driven stability assessments across a wide range of drug modalities.
Service at MtoZ Biolabs
Cryo-EM allows direct observation of particle size, morphology, encapsulation, aggregation, and structural integrity under near-native conditions. Through MtoZ Biolabs' Drug Stability Testing Service, Cryo-EM enables comprehensive structural evaluation to support stability studies, including:
✔️Detection of Morphological Changes: Identifying vesicle deformation, capsid disruption, or nanoparticle aggregation during storage.
✔️Evaluation of Encapsulation Stability: Assessing payload leakage or payload structural changes within carriers over time.
✔️Monitoring Aggregation and Fragmentation: Visualizing early aggregation events or particle breakdown.
✔️Comparative Analysis Under Stress Conditions: Supporting accelerated stability studies by comparing samples before and after thermal, mechanical, or chemical stress.
✔️Batch Consistency Monitoring: Verifying structural uniformity across stability batches and production lots.
These structural insights directly inform formulation optimization, shelf-life prediction, storage condition determination, and regulatory stability reporting.
Analysis Workflow
1. Consultation and Stability Study Design: Collaborative planning to define drug stability testing protocols, and target critical quality attributes.
2. Sample Preparation and Vitrification: Samples are vitrified under optimized conditions to preserve native structure without introducing artifacts.
3. Cryo-EM Imaging and Data Collection: High-resolution images are acquired using direct electron detectors under low-dose conditions to protect sample integrity.
4. Structural Analysis and Stability Assessment: Comprehensive analysis includes particle morphology, size distribution, aggregation profiling, encapsulation integrity, and comparative evaluation across timepoints.
5. Reporting and Interpretation: Clients receive detailed reports with representative images, quantitative structural metrics, and scientific interpretation to support stability study conclusions.
Service Advantages
☑️Flexible Study Design
We customize stability protocols to match client-specific formulations, target storage conditions, and regulatory requirements, adapting to real-time or accelerated study needs.
☑️Rapid and Reliable Turnaround
We maintain efficient project timelines from sample preparation to data delivery, helping clients meet critical decision points during stability program execution.
☑️Expert Guidance and Consultation
Our multidisciplinary team offers technical support throughout the project, helping clients interpret structural findings and optimize formulations based on real data.
☑️One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Applications
1. Early-Stage Formulation Stability Screening
We support early formulation development by evaluating nanoparticle morphology, payload retention, and aggregation tendency, helping identify stable formulation candidates before scale-up.
2. Real-Time Stability Monitoring
Through periodic Cryo-EM analysis during real-time stability studies, we visualize structural changes and confirm the maintenance of critical quality attributes over shelf-life.
3. Comparability Studies After Process Changes
Our service enables structural verification between pre-change and post-change batches, supporting regulatory comparability requirements during manufacturing modifications.
4. Storage Condition Evaluation and Stability Optimization
We provide detailed structural assessments under various storage conditions, helping clients verify product stability, select appropriate storage strategies, and support quality assurance throughout the product lifecycle.
Case Study
Cryo-EM Characterization of π-Electron Stabilized Micellar Systems
In this sudy, a novel polyelectrolyte block copolymer micelle system (dPGS-SS-POxPPh-Py) was developed to enhance stability for drug delivery applications. The micelles exhibited a very low critical micelle concentration (0.3 mg/L) and enabled efficient encapsulation of Docetaxel (DTX) with a loading capacity of up to 13 wt%. Cryogenic electron microscopy (Cryo-EM) confirmed the spherical morphology of the micelles and measured particle sizes of 57 nm (unloaded) and 80 nm (loaded). Structural insights from Cryo-EM, alongside DLS and spectroscopy analyses, demonstrated the significant role of internal π–π interactions in maintaining micelle integrity. Stability was further validated by incubation in human serum proteins, showing no size increase or disassembly over four days. These results underscore the value of Cryo-EM in assessing morphological stability and structural robustness of advanced micellar drug delivery systems.
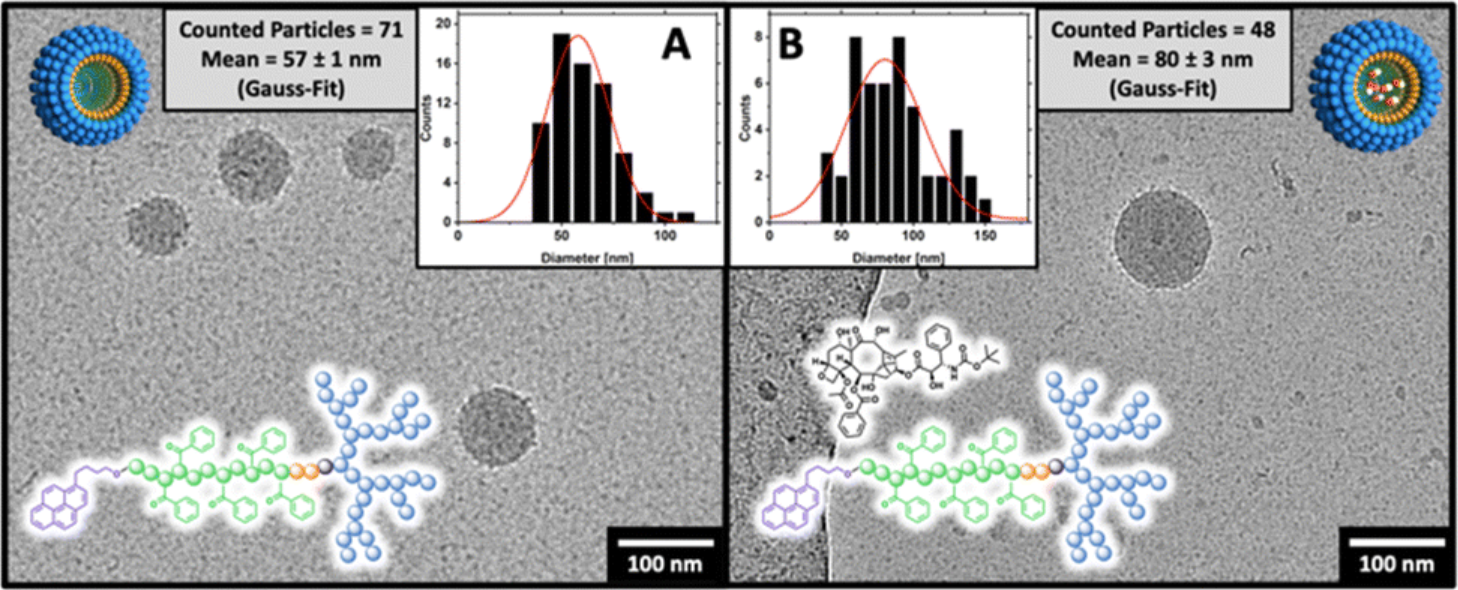
Figure 1. Cryo-TEM Images of (A) Empty and (B) Docetaxel-Loaded dPGS8.6-SS-POxPPh7.9-Py Micelles
FAQ
Q: How does Cryo-EM help in stability testing?
Cryo-EM allows direct visualization of particle morphology, encapsulation integrity, and aggregation behavior over time, providing critical data to monitor structural stability under real-time or stress conditions.
MtoZ Biolabs empowers drug developers with cutting-edge Cryo-EM stability testing solutions to ensure product success from development through commercialization. To learn more about our Drug Stability Testing Service using Cryo-EM, or to discuss your specific stability study requirements, please contact us.
How to order?







