Drug Manufacturing Process Validation Service | Cryo-EM
In modern pharmaceutical development, ensuring the consistency, integrity, and reproducibility of drug manufacturing processes is essential for regulatory approval and product quality assurance. In the production of complex biologics, gene therapies, and vaccine vectors, cryogenic electron microscopy (Cryo-EM) offers critical structural information under near-native conditions. By revealing detailed morphological and structural information, Cryo-EM provides actionable insights that support process optimization, method validation, and final product quality assurance.
MtoZ Biolabs offers a dedicated Drug Manufacturing Process Validation Service utilizing Cryo-EM technology, helping pharmaceutical and biotechnology companies strengthen process control, support regulatory submissions, and accelerate the development of high-quality therapeutic products.
Service at MtoZ Biolabs
MtoZ Biolabs' Cryo-EM-based Drug Manufacturing Process Validation Service enables direct imaging of pharmaceutical particles, aggregates, nanoparticles, and macromolecular assemblies under near-native conditions. Our services include:
✔️Morphological Assessment: Visualization of particle size, shape, lamellarity, and surface features without artifacts.
✔️Aggregate and Impurity Detection: Identification and quantification of subvisible aggregates or contaminants at nanoscale resolution.
✔️Encapsulation and Loading Validation: Structural verification of payload encapsulation efficiency and uniformity.
✔️Batch-to-Batch Comparability: Quantitative comparison of structural attributes across production batches.
✔️Stability Monitoring: Assessment of structural changes under storage, stress, or formulation modification conditions.
These insights are crucial for confirming that manufacturing processes consistently produce products meeting predefined specifications.
Analysis Workflow
1. Consultation and Project Scoping
We collaborate with clients to define critical quality attributes, target formulations, and validation objectives.
2. Sample Preparation and Vitrification
Samples are vitrified using optimized plunge-freezing or grid preparation protocols to preserve native structures.
3. Cryo-EM Imaging and Data Collection
High-resolution imaging is performed under low-dose conditions to capture detailed structural information.
4. Structural Analysis and Quality Assessment
Analysis includes particle sizing, morphology evaluation, aggregation profiling, encapsulation verification, and batch-to-batch comparability studies.
5. Reporting and Regulatory Support
Clients receive detailed reports with quantitative metrics, representative micrographs, and structural assessments suitable for internal validation or regulatory submission.
Service Advantages
1. Tailored Project Planning
We work closely with clients to define validation objectives, critical quality attributes, and analysis parameters, ensuring each project is aligned with manufacturing and regulatory needs.
2. End-to-End Support
From sample preparation and vitrification to imaging, analysis, and reporting, we provide a complete workflow, eliminating the need for clients to coordinate multiple vendors or technical steps.
3. Flexible Service Models
We offer flexible service models that accommodate early-stage development, process optimization, and manufacturing validation, adapting to project timelines and technical requirements.
4. Multidisciplinary Expertise Integration
Our team combines knowledge in cryo-EM imaging, pharmaceutical manufacturing, regulatory science, and quality systems, ensuring that each validation study is scientifically rigorous.
Applications
1. Biologics and Biosimilars Manufacturing
Our service supports structural characterization and batch comparability assessments of protein therapeutics, monoclonal antibodies, and biosimilar products, ensuring morphological consistency and aggregation control during manufacturing validation.
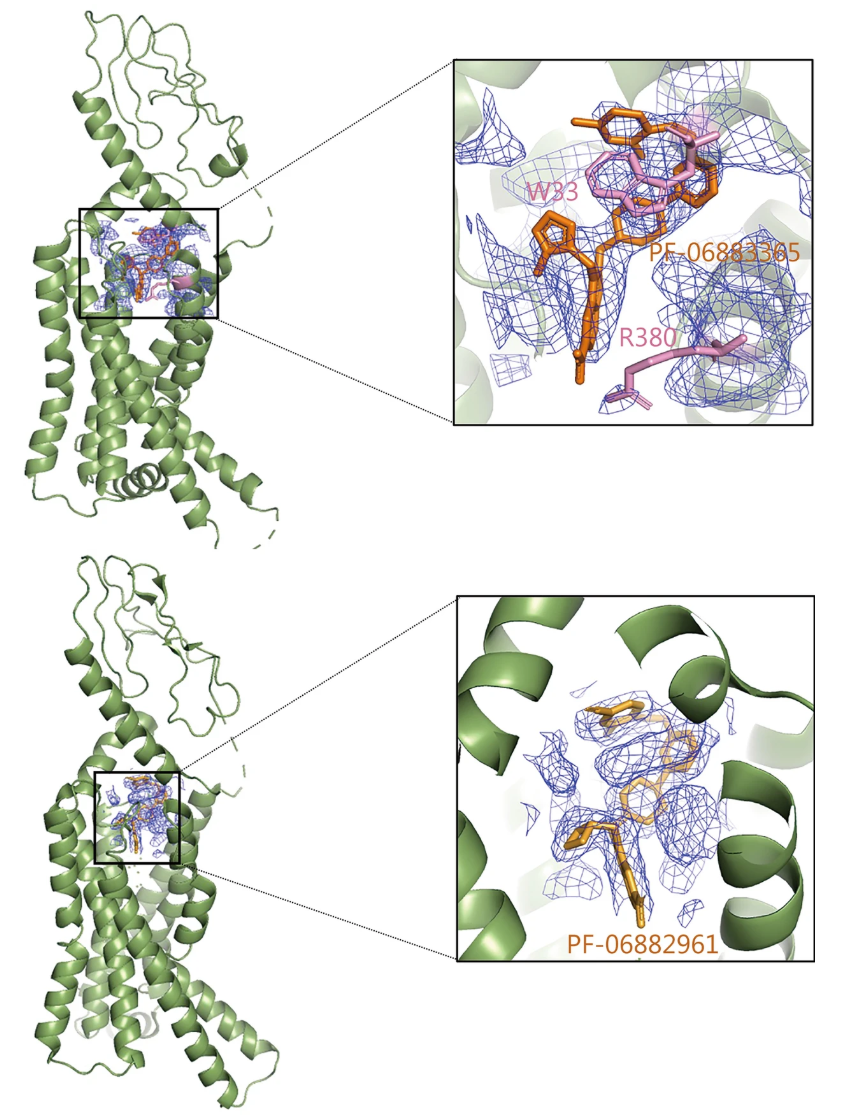
Figure 1. Application of Cryo-EM in Structure-Based Drug Design
2. Nanoparticle-Based Drug Delivery Systems
We provide direct visualization and quality attribute analysis for lipid nanoparticles (LNPs), polymeric nanoparticles, and hybrid delivery platforms used in gene therapy, mRNA vaccines, and targeted drug delivery applications.
3. Liposomal and Micellar Formulations
Our Cryo-EM validation service evaluates size distribution, membrane integrity, encapsulation efficiency, and morphological stability of liposome- and micelle-based drug products across production batches.
4. Viral Vector Manufacturing for Gene Therapy
We assist in verifying capsid structure, genome packaging efficiency, and particle uniformity for recombinant AAVs, lentiviral vectors, and other viral-based delivery systems, supporting process consistency and regulatory compliance.
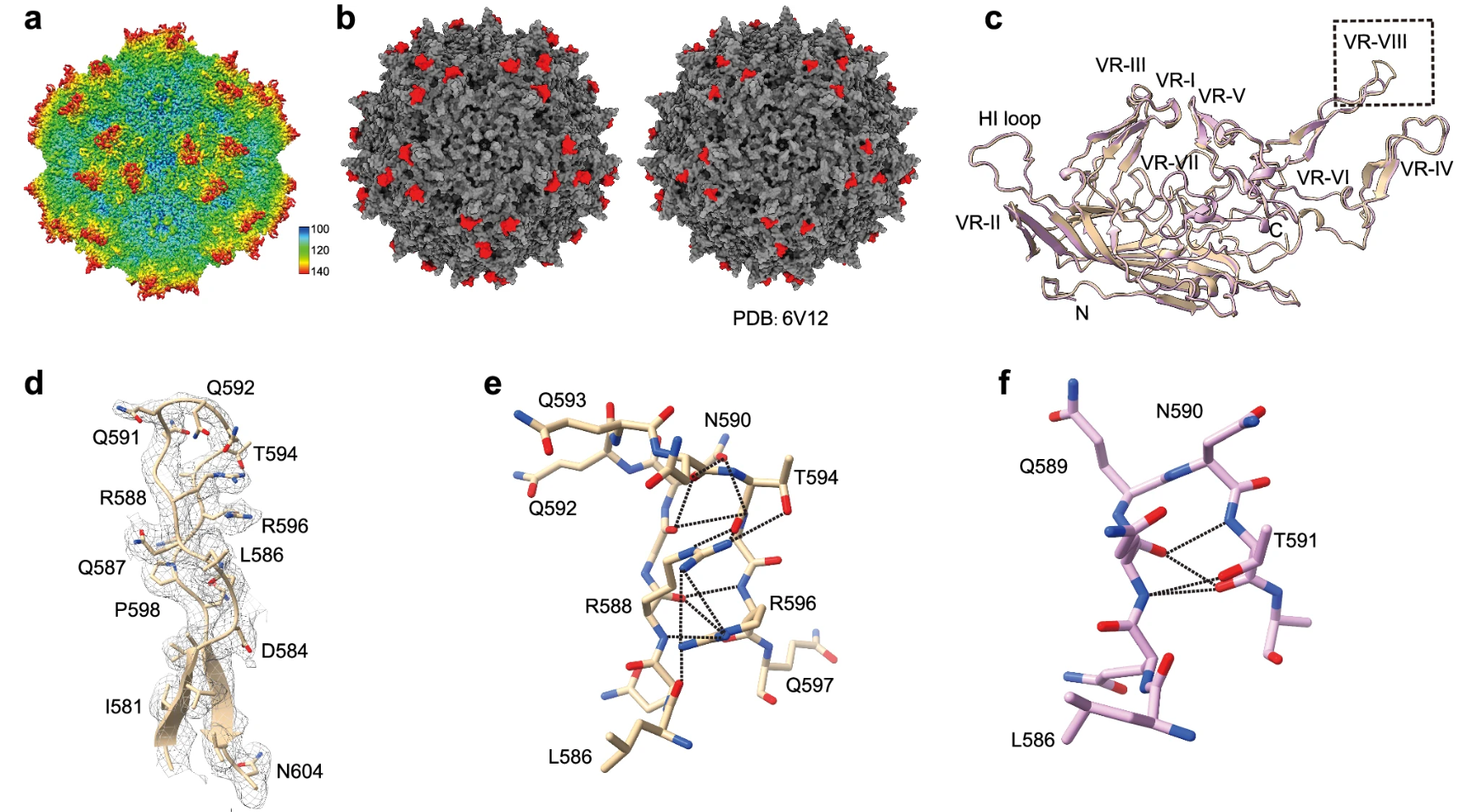
Figure 2. Cryo-EM Reveals Differences between AAVv128 and AAV8
5. Exosome and Extracellular Vesicle Products
Our service enables structural analysis of natural or engineered vesicles intended for therapeutic use, providing critical validation data for morphology, purity, and vesicle integrity throughout the manufacturing process.
6. Stability and Stress Testing Studies
We support stability testing by monitoring structural changes of nanoparticles, biologics, and delivery systems under storage and stress conditions, aiding shelf-life validation and formulation optimization.
FAQ
Q1: Can Cryo-EM detect subtle structural differences between manufacturing batches?
A1: Yes. Cryo-EM enables comparison of particle size, morphology, aggregation states, and encapsulation features across batches, supporting comparability assessments during process validation.
Q2: What types of drug products are suitable for your Cryo-EM validation service?
A2: We support a broad range of products, including biologics, biosimilars, vaccines, lipid nanoparticles (LNPs), polymeric nanoparticles, liposomes, micelles, viral vectors, and exosome-based therapeutics.
MtoZ Biolabs is committed to enabling robust, data-driven manufacturing process validation through state-of-the-art cryo-EM solutions. To learn more about our Drug Manufacturing Process Validation Service using Cryo-EM, or to discuss your specific validation needs, please contact the MtoZ Biolabs technical team.
How to order?







