DNA/RNA Drug Sequence Confirmation Service
- Drug Candidate Screening and Development
- Quality Control and Batch Consistency
- Pharmacokinetics and Pharmacodynamics Studies
- Formulation and Delivery System Optimization
DNA/RNA Drug Sequence Confirmation Service is a specialized analytical service that precisely verifies and confirms the primary sequence and chemical modifications of synthetic DNA or RNA drugs. The core objective of this service is to ensure that the nucleotide arrangement of nucleic acid drugs is fully consistent with the designed sequence, while also confirming the integrity of chemical modifications and excluding truncated fragments, base mismatches, or degradation byproducts that may arise during synthesis or storage.
With the rapid development of nucleic acid drugs such as antisense oligonucleotides and siRNA, DNA/RNA drugs have become an important part of the biopharmaceutical field. The activity and function of DNA/RNA drugs are highly dependent on the accuracy of their nucleotide sequences and chemical modifications. Even a single nucleotide mutation, deletion, or modification error may cause drug failure or adverse reactions. During drug development and production, synthesis often results in truncated fragments, mismatches, modification losses, or degradation byproducts, making comprehensive and precise sequence confirmation essential for ensuring drug quality and safety.
Services at MtoZ Biolabs
MtoZ Biolabs provides DNA/RNA Drug Sequence Confirmation Service to ensure the accuracy and integrity of nucleic acid drug sequences. Using multiple platforms such as chromatography, mass spectrometry, and capillary electrophoresis, our service covers molecular weight determination, fragment ion analysis, sequence alignment, and modification verification, enabling the identification of truncated fragments, impurities, and modification losses to provide comprehensive support for drug development and quality control. At the same time, MtoZ Biolabs integrates advanced bioinformatics analysis with standardized data processing workflows to generate complete validation reports, helping research and pharmaceutical clients obtain reliable data for candidate drug evaluation, production quality control, and preclinical studies.
Analysis Workflow
The general workflow of DNA/RNA Drug Sequence Confirmation Service is as follows:
1. Sample Receipt and Quality Assessment
After submission, DNA/RNA drug samples are first checked for purity and integrity to ensure they meet the basic requirements for subsequent analyses.
2. Separation and Pretreatment
Chromatography is used to remove salts and impurities and to separate the target product from truncated fragments or byproducts.
3. Mass Spectrometry Detection
High-resolution mass spectrometry is applied to determine the exact molecular weight of oligonucleotides, providing preliminary confirmation of overall sequence accuracy.
4. Fragment Ion Analysis
Characteristic fragment ions are obtained to verify nucleotide arrangement base by base and to locate common modifications.
5. Data Comparison and Bioinformatics Analysis
Experimental spectra are compared with the designed sequence to confirm sequence coverage, modification distribution, and potential variations.
6. Report Generation
A complete sequence confirmation report is provided, including molecular weight determination, fragment spectrum analysis, and modification verification.
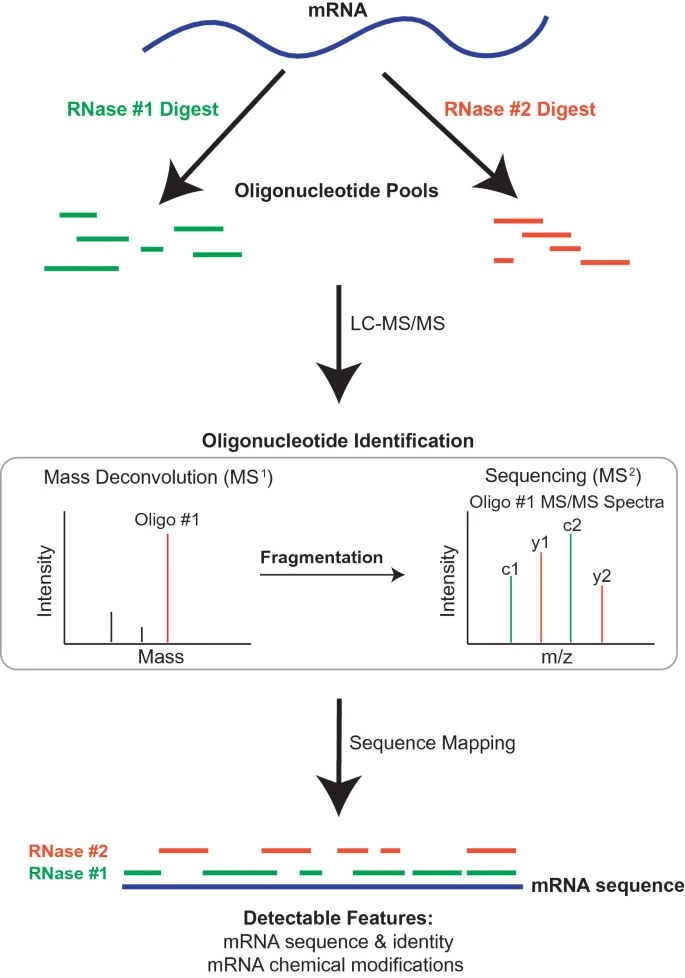
Corrêa IR. et al. RNA Technologies, vol 14. 2023.
Figure 1. A general workflow for RNA sequence mapping by LC-MS/MS.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Sequence Confirmation Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all DNA/RNA Drug Sequence Confirmation data, providing clients with a comprehensive data report.
Precise Identification of Modification Sites: Confirm the presence and localization of common chemical modifications, such as phosphorothioate, to ensure drug functionality and stability.
Customization and Data Security: Design flexible solutions tailored to specific project requirements and strictly enforce data confidentiality agreements to protect clients’ intellectual property and research outcomes.
Sample Submission Suggestions
Sample Types
Acceptable samples include synthetic DNA, RNA, and their modified forms as DNA/RNA drugs.
Storage and Transportation
Recommended storage at -20°C or below, with shipment on dry ice during transportation.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Verify consistency between the synthetic sequence and the designed sequence to avoid research deviations.
Monitor production processes and support product release.
Ensure sequence accuracy of nucleic acid drugs used in in vivo and in vitro experiments.
Confirm modification and sequence stability to support delivery system design.
FAQ
Q1: Can Base-by-Base Coverage Be Achieved, Especially for Long-Chain RNA?
A1: Yes. By combining high-resolution LC-MS/MS with CID/HCD/ETD fragmentation modes, base-by-base coverage can be achieved for short oligonucleotides. For long-chain RNA over 100 nt, a strategy combining segmented enzymatic digestion with fragment ion analysis is typically used to improve coverage.
Q2: Can DNA/RNA Drug Sequence Confirmation Service Accurately Detect and Localize Common Modifications?
A2: Yes. This service can detect and localize common modifications such as 2'-O-methylation, phosphorothioate, PS bonds, and cap structures. Optimized chromatographic separation and negative-ion ESI mode improve resolution for modification site determination.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
DNA/RNA Drug Identification Service
DNA/RNA Drug Structural Characterization Service
DNA/RNA Drug Secondary Structures Analysis Service
How to order?







