DNA/RNA Drug Secondary Structures Analysis Service
- Sample Types
- Storage and Transportation
DNA/RNA Drug Secondary Structures Analysis Service refers to a service that systematically detects and analyzes the secondary structures formed by DNA and RNA drugs under physiological or near-physiological conditions using multiple approaches such as spectroscopy, mass spectrometry, nuclear magnetic resonance (NMR), and computational prediction. The purpose is to confirm that nucleic acid drugs maintain their intended conformations during design, synthesis, and application, to evaluate stability and functional relevance, and to provide scientific support for drug development, quality control, and preclinical research.
DNA and RNA are not merely linear nucleotide sequences; under physiological and experimental conditions, they can form diverse secondary structures through hydrogen bonding and base stacking interactions, including hairpins, stem-loops, bulges, internal loops, and G-quadruplexes. These structures play essential roles in gene expression regulation, molecular recognition, drug binding, and stability maintenance. For nucleic acid drugs such as antisense oligonucleotides, siRNA, mRNA vaccines, and aptamers, secondary structures directly affect stability, delivery efficiency, binding specificity, and immunogenicity, making secondary structure analysis particularly important for drug design optimization, quality control, and functional validation.
Services at MtoZ Biolabs
Leveraging high-resolution mass spectrometry platforms, circular dichroism (CD), nuclear magnetic resonance (NMR), and advanced computational prediction and thermodynamic analysis methods, MtoZ Biolabs provides DNA/RNA Drug Secondary Structures Analysis Service to systematically characterize the secondary structural features of nucleic acid drugs under near-physiological conditions.
Our service covers global conformation detection as well as detailed analysis of local structures, enabling the identification of diverse forms such as hairpins, stem-loops, and G-quadruplexes, while also evaluating their stability and functional relevance. By integrating multiple analytical platforms with bioinformatics-based data analysis, MtoZ Biolabs delivers high-quality results that meet both scientific and industrial needs, supporting drug design optimization, quality control, and preclinical validation.
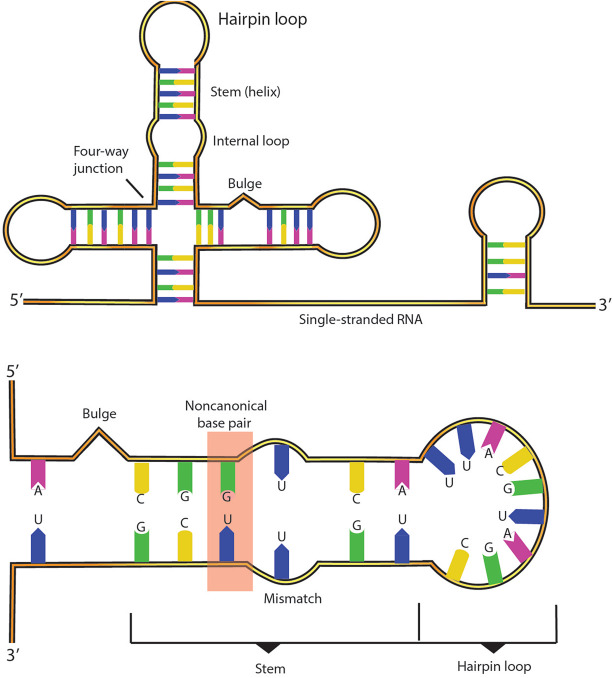
Shen CH. Diagnostic molecular biology. Academic Press. 2019.
Figure 1. Schematic diagram of the typical structural motifs in an RNA secondary structure.
Analysis Workflow
The general workflow of DNA/RNA Drug Secondary Structures Analysis Service is as follows:
1. Sample Preparation and Quality Assessment
Assess sample purity and concentration to ensure suitability for subsequent analysis.
2. Experimental Detection
Apply multiple experimental methods such as CD, NMR, and MS to obtain data related to secondary structures and thermal stability.
3. Computational Simulation
Perform structure prediction based on thermodynamics and base-pairing rules to generate possible secondary structure models.
4. Data Comparison and Validation
Cross-validate experimental results with computational predictions to improve the accuracy of structural analysis.
5. Functional Analysis
Evaluate the roles of secondary structures in stability, binding specificity, and drug function according to research objectives.
6. Result Integration and Report Generation
Provide a complete report that includes experimental results, predicted models, and functional analysis.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced DNA/RNA Drug Secondary Structures Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all DNA/RNA Drug Secondary Structures Analysis data, providing clients with a comprehensive data report.
Customized Analytical Solutions: Design personalized experiments and analysis workflows based on client research objectives.
Compliance and Data Security: Provide standardized reports that meet the requirements of scientific research and drug development, while strictly implementing confidentiality agreements to ensure intellectual property and data security.
Sample Submission Suggestions
Applicable to synthetic DNA, RNA, or modified oligonucleotides as DNA/RNA drugs.
Recommended storage at low temperature (-20°C or below), with shipment on dry ice during transportation, avoiding repeated freeze-thaw cycles.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Gene Function and Regulation Studies: Reveal the role of secondary structures in transcription and translation mechanisms.
Drug Design and Development: Optimize the stability and efficacy of antisense oligonucleotides, siRNA, and mRNA vaccines.
Quality Control and Batch Consistency: Confirm conformational consistency of nucleic acid drugs during production.
Biotechnology and Synthetic Biology: Guide the design and application of synthetic nucleic acid molecules and expand their potential applications in materials science and molecular engineering.
FAQ
Q1: Can DNA/RNA Drug Secondary Structures Analysis Service Evaluate the Impact of Modifications on Secondary Structures?
A1: Yes. Common modifications such as 2'-O-methylation, phosphorothioate (PS) bonds, and thiolation can be precisely detected using mass spectrometry and NMR, and their effects on secondary structure stability and conformation can be evaluated through melting curve analysis or thermodynamic studies.
Q2: How Is Result Reliability Ensured Across Multiple Platforms?
A2: Each method has its own advantages: CD spectroscopy is suitable for global conformation analysis, NMR provides local structural resolution, mass spectrometry detects conformational isomers, and computational prediction offers theoretical models. Cross-validation between experimental data and predictions enhances both accuracy and reproducibility of the results.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
DNA/RNA Drug Structural Characterization Service
DNA/RNA Drug Sequence Confirmation Service
DNA/RNA Drug Higher-Order Structure Analysis Service
How to order?







