Differences Between Immunoprecipitation and Co-Immunoprecipitation
-
A specific antibody is used to recognize and bind the target protein.
-
The antibody-protein complex is captured using protein A/G magnetic beads or agarose beads.
-
After elution, downstream analyses are performed, such as Western blotting, SDS-PAGE, or mass spectrometry.
-
Purification of a single protein for subsequent functional characterization.
-
Enrichment of low-abundance proteins from complex samples.
-
Analysis of protein post-translational modifications (e.g., phosphorylation and ubiquitination).
-
Sample preparation prior to protein identification and quantitative analysis.
-
The primary goal of Co-IP is not the isolation of protein A itself, but the confirmation or identification of proteins that interact with it.
-
For non-covalent, transient, or weak protein-protein interactions, Co-IP is highly sensitive to experimental conditions, including buffer composition and washing stringency.
-
Validation of protein-protein interactions (PPIs).
-
Investigation of upstream and downstream relationships within signaling pathways.
-
Interactome analysis in combination with mass spectrometry.
-
Identification of potential molecular targets of pharmacological agents.
In life science research, elucidating how proteins cooperate with one another is fundamental to understanding key biological processes, including signal transduction, transcriptional regulation, and disease mechanisms. Immunoprecipitation (IP) and co-immunoprecipitation (Co-IP) are classical experimental approaches for investigating protein interaction networks and are widely applied in proteomics and cell signaling studies. Despite their similar nomenclature and partially overlapping experimental workflows, these two techniques differ substantially in their experimental objectives, underlying principles, and modes of data interpretation.
What Is Immunoprecipitation (IP)?
Immunoprecipitation is a technique that employs specific antibodies to selectively enrich a target protein from complex biological samples.
1. Basic Principle
2. Application Scenarios
What Is Co-Immunoprecipitation (Co-IP)?
Co-immunoprecipitation is an extension of immunoprecipitation that is designed to investigate interactions between a target protein and its associated binding partners.
1. Core Logic
If protein A forms a complex with protein B within cells, immunoprecipitation of protein A using an anti-A antibody will result in the simultaneous co-precipitation of protein B.
2. Key Points
3. Application Scenarios
Core Differences Between IP and Co-IP
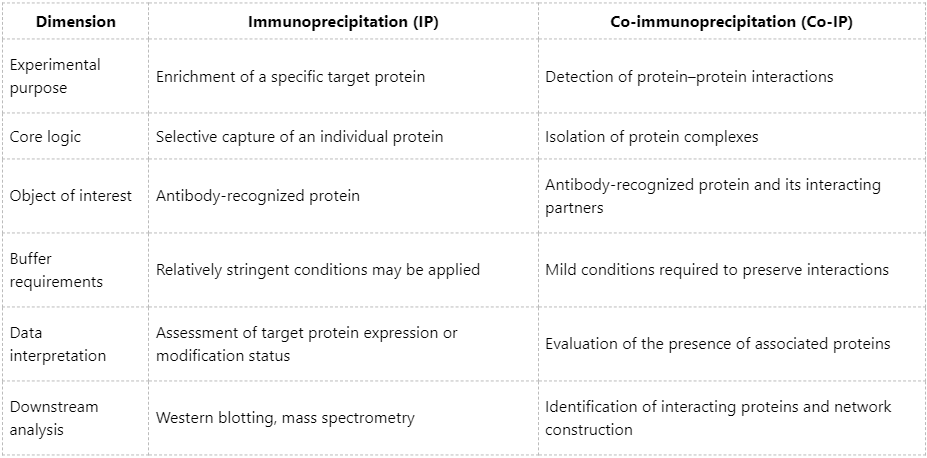
Overall, immunoprecipitation primarily focuses on the selective isolation of a defined protein of interest, whereas co-immunoprecipitation is aimed at uncovering protein interaction relationships. The two techniques are complementary and should be selected according to specific research objectives.
Co-IP Combined With Mass Spectrometry: A Powerful Strategy for Interaction Network Analysis
With advances in high-resolution mass spectrometry, the combination of co-immunoprecipitation and mass spectrometry (Co-IP-MS) has become an effective strategy for systematic analysis of protein interaction networks.
At MtoZ Biolabs, a standardized Co-IP-MS workflow has been established by integrating a high-specificity antibody screening platform with the Orbitrap Exploris™ 480 mass spectrometry system, enabling:
1. Parallel processing of multiple samples.
2. In-depth characterization of protein interaction profiles.
3. Quantitative assessment of interaction abundance and confidence.
4. Visualization of protein interaction networks.
This approach is applicable not only to basic research, such as the identification of regulatory factors associated with transcription factors, but also to translational studies, including novel drug target discovery and disease biomarker development.
Technical Selection Recommendations: When to Use IP or Co-IP?
The choice between IP and Co-IP should be guided by the research goal:
1. To enrich a specific protein for quantitative or post-translational modification analysis: immunoprecipitation (IP) is appropriate.
2. To determine whether a protein interacts with another protein: co-immunoprecipitation (Co-IP) is preferred.
3. To systematically explore protein interaction networks and identify potential regulatory factors: Co-IP combined with LC-MS/MS is recommended.
In practice, IP and Co-IP can also be applied sequentially. For example, a target protein may first be enriched by IP and subsequently analyzed for interaction partners, enabling comprehensive investigation from protein identity to interaction mechanisms.
Immunoprecipitation and co-immunoprecipitation are indispensable methodologies in proteomics research. In many cases, careful optimization of experimental conditions is more critical to data reliability and scientific value than the choice of technique alone.
At MtoZ Biolabs, in addition to standardized IP and Co-IP services, integrated solutions for interactome studies are provided, including:
1. Antibody screening and validation.
2. Experimental optimization and customization.
3. High-throughput mass spectrometry-based identification.
4. Bioinformatics analysis and interaction network construction.
Researchers are welcome to contact MtoZ Biolabs for further information on protein interaction research services and individualized experimental design support.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







