Dextrin Analysis Service | Pharmaceutical Excipient
Dextrin Analysis Service is a systematic analytical service for evaluating the physicochemical properties, structural characteristics, and functional performance of dextrin. The service covers component identification, molecular structure analysis, thermal behavior assessment, and biocompatibility testing, aiming to provide reliable data support and quality assurance for drug development and formulation optimization.
Dextrin is a class of polysaccharide derivatives composed of glucose units linked mainly by α-1,6 glycosidic bonds with branched structures. Due to its good solubility, thermal stability, and biocompatibility, dextrin is widely used in the development of pharmaceutical excipients. Systematic physicochemical and structural analysis of dextrin not only helps to reveal its composition, molecular conformation, and thermal characteristics but also allows evaluation of its stability, dissolution behavior, and safety in pharmaceutical formulations, thereby providing a scientific basis for drug research and manufacturing.
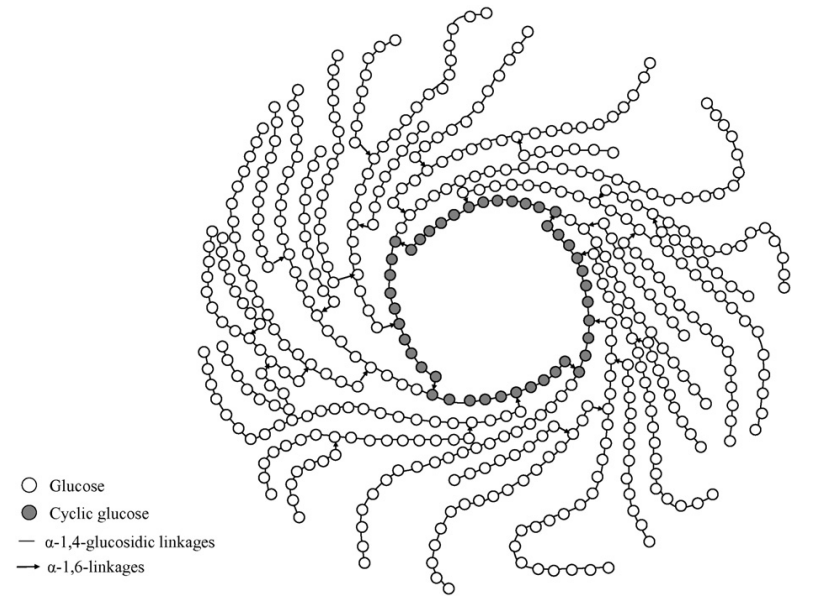
Kadota K. et al. Eur J Pharm Sci. 2015.
Figure 1. Schematic representation of a highly branched cyclic dextrin
Services at MtoZ Biolabs
MtoZ Biolabs provides a systematic Dextrin Analysis Service covering comprehensive testing from physicochemical properties to biological evaluation, supporting quality control throughout the entire process of drug development and manufacturing.
1. Quantitative Analysis
High-performance liquid chromatography (HPLC) and Fourier-transform infrared spectroscopy (FTIR) are used to accurately determine the content of dextrin.
2. Structural Characterization
Nuclear magnetic resonance (NMR) and electron microscopy are applied to analyze the molecular structure, morphological features, and crystallinity of dextrin.
3. Thermal Performance Study
Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) are employed to evaluate the melting point, thermal stability, and thermal decomposition behavior of dextrin.
4. Physical Property Testing
Particle size distribution and specific surface area measurements are performed to reveal their influence on solubility and stability.
5. Biological Evaluation
Degradability and cytotoxicity tests are provided to assess the safety and applicability of dextrin in formulations.
Service Advantages
1. Advanced Analysis Platform
MtoZ Biolabs established an advanced Dextrin Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. Multi-Level Comprehensive Characterization Capability
Systematically analyzes dextrin characteristics from multiple perspectives, including chemical composition, molecular structure, thermal behavior, physical properties, and biocompatibility, to achieve a complete understanding of excipient performance.
3. Customized Analytical Solutions
Flexibly selects and combines testing methods according to the client’s research goals and experimental requirements, tailoring the most suitable analytical process and study plan.
Sample Submission Suggestions
1. Sample Types
Powdered or solution forms of dextrin samples can be submitted. If there are any special modifications or sources, please provide detailed information at the time of submission.
2. Storage and Transportation
Samples should be stored in sealed containers under dry conditions, and low-temperature or dry ice conditions are recommended during transportation to prevent degradation.
It is recommended to contact the MtoZ Biolabs technical team prior to sample submission to obtain detailed and tailored guidelines for sample preparation and submission.
Applications
Application examples of Dextrin Analysis Service:
1. Pharmaceutical Formulation Optimization
By systematically evaluating the solubility, thermal stability, and structural properties of dextrin, the service supports formulation design and prescription improvement, enhancing drug dissolution rate, stability, and bioavailability.
2. Drug Delivery System Development
Through particle size distribution, specific surface area, and biocompatibility analysis, dextrin is validated for its applicability and safety in novel drug carriers such as sustained-release formulations.
3. Process and Storage Stability Studies
Thermal and degradation analyses reveal the stability and decomposition behavior of dextrin under different temperatures, humidity, and processing conditions.
4. Cross-Disciplinary Applications
Beyond pharmaceuticals, the analytical results can also support applications in food, fermentation, and biomaterials, expanding the potential of dextrin as a functional excipient or raw material.
How to order?







