Combinational Applications of Cryo-EM and Mass Spectrometry
The combined application of cryo-electron microscopy (Cryo-EM) and mass spectrometry (MS) is an integrated research strategy that merges structural analysis with molecular composition identification. Cryo-EM provides three-dimensional morphology and assembly structures of macromolecular complexes in a near-native state, while MS enables precise qualitative and quantitative analysis of constituent proteins, post-translational modifications (such as phosphorylation and ubiquitination), and bound small molecules. Together, they complement each other’s blind spots and enhance the comprehensiveness and accuracy of complex system characterization.
Combinational applications of Cryo-EM and mass spectrometry are widely used in the study of systems such as viral particles, ribosomes, membrane protein complexes, protein assemblies, and organelles. This approach is especially suitable for samples with high conformational heterogeneity or limited purity. By first obtaining the overall structural framework through Cryo-EM and then using MS to identify subunit composition, modification types, and binding ligands, researchers can achieve a more complete understanding of molecular mechanisms and structure–function relationships, supporting novel target discovery, drug mechanism research, and systems biology exploration.
Services at MtoZ Biolabs
Based on advanced cryo-electron microscopy and high-resolution mass spectrometry systems, the combinational applications of Cryo-EM and mass spectrometry service provided by MtoZ Biolabs offer dual capabilities for structural and compositional analysis of samples. Cryo-EM is used to visualize the three-dimensional configuration and assembly state of biological macromolecules, while mass spectrometry deciphers their molecular composition, sequence features, and modification sites. The integration of both techniques enables comprehensive analysis from structure to composition. This service is well-suited for analyzing biologically heterogeneous and compositionally complex samples, helping to overcome the limitations of individual techniques and providing systematic structural and compositional data support for molecular mechanism studies, drug target validation, and vector construction.
Analysis Workflow
1. Sample Preparation and Condition Optimization
Samples are purified and buffer conditions adjusted according to research objectives to ensure compatibility with both Cryo-EM and mass spectrometry platforms.
2. Cryo-EM Imaging and Data Acquisition
Samples are rapidly vitrified and imaged under low-dose conditions using Cryo-EM to obtain high-resolution 2D images and 3D structural data.
3. Mass Spectrometry Analysis and Component Identification
Mass spectrometry techniques are employed for protein identification, post-translational modification analysis, or assessment of protein complex assembly states.
4. Data Integration and Structural Annotation
Protein information obtained from mass spectrometry is integrated with Cryo-EM 3D structures to build atomic models, identify functional regions, or annotate heterogeneous conformations.
Service Advantages
1. Complementary Structural and Compositional Information
Cryo-EM provides high-resolution three-dimensional structural data, while mass spectrometry complements with molecular composition and modification status, enabling comprehensive analysis of complex systems.
2. Improved Structural Annotation Accuracy
Protein sequences and modification features identified by mass spectrometry can be accurately mapped onto Cryo-EM density maps, enhancing the reliability of subunit localization and functional domain interpretation.
3. Support for Heterogeneous System Analysis
Applicable to systems with high structural heterogeneity or dynamic assemblies, enabling differentiation of conformational states, compositional variants, and ligand-binding scenarios.
4. Facilitates High-Throughput Screening
Coupled with automated data acquisition and analysis workflows, this approach enables rapid screening of representative conformations and compositional features across multiple samples, accelerating research progress.
Applications
1. Structural Analysis of Protein Complexes
By integrating Cryo-EM-based 3D structural modeling with mass spectrometry-based subunit identification, the assembly modes and interaction networks of multi-subunit complexes can be thoroughly analyzed.
2. Dynamic Conformation Studies
Combinational applications of Cryo-EM and mass spectrometry enable the analysis of proteins or complexes with multiple conformational states, helping to reveal the correlation between conformational changes and functional states.
3. Post-Translational Modification Site Validation
Post-translational modifications (e.g., phosphorylation, acetylation) identified by mass spectrometry can be spatially mapped using Cryo-EM density maps to clarify their structural impact.
4. Virus Particle and Vaccine Research
Combinational applications of Cryo-EM and mass spectrometry can be used to evaluate viral capsid structure and compositional integrity, supporting vaccine component identification and consistency assessment through complementary mass spectrometry analysis.
Case Study
1. Combining Cryo-Electron Microscopy (cryo-EM) and Cross-Linking Mass Spectrometry (CX-MS) for Structural Elucidation of Large Protein Assemblies
This study aims to explore the advantages and practical applications of combining cryo-electron microscopy (cryo-EM) with cross-linking mass spectrometry (CX-MS) in resolving the structures of large macromolecular protein complexes. The research focuses on several large heteromeric protein assemblies. The authors first performed cross-linking reactions to obtain spatial constraint information, followed by cryo-EM for three-dimensional reconstruction and integrative modeling. The results showed that the cross-linking sites provided by CX-MS significantly improved the accuracy and atomic-level completeness of the cryo-EM reconstructed models, particularly enhancing modeling in low-resolution regions. The study demonstrates that this integrative strategy offers notable advantages in systems with high structural heterogeneity, dynamic flexibility, or lacking high-resolution crystallographic data, enabling more reliable structural modeling and functional interpretation.
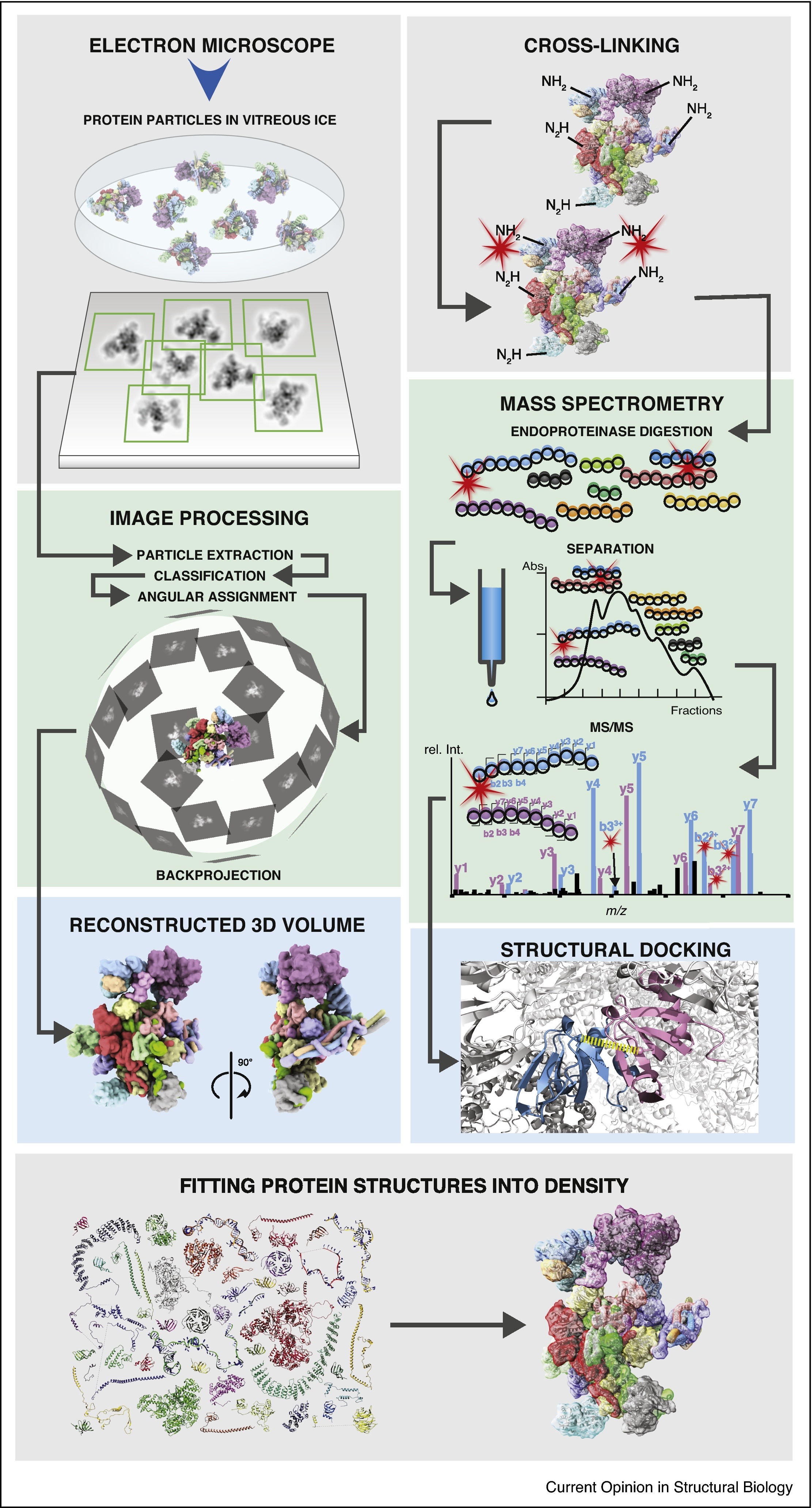
Schmidt, C. et al. Current Opinion in Structural Biology, 2017.
Figure 1. Schematic Overview of Workflows of Single Particle Cryo-EM (left) and CX-MS (right).
2. Complementarity of Hydrogen/Deuterium Exchange Mass Spectrometry and Cryo-Electron Microscopy
This study aims to evaluate the complementarity between hydrogen/deuterium exchange mass spectrometry (HDX-MS) and cryo-electron microscopy (Cryo-EM) in elucidating the structure and dynamics of protein complexes. The research targets several large macromolecular assemblies with flexible regions. The authors used HDX-MS to obtain information on protein dynamics and solvent accessibility in solution and integrated these data with Cryo-EM-derived 3D structures to compare their respective capabilities in identifying flexible regions and conformational heterogeneity. The results indicate that HDX-MS compensates for Cryo-EM’s limitations in low-resolution areas by enhancing dynamic structural insights, while Cryo-EM provides spatial information on overall conformations and interaction sites. The study concludes that the synergistic application of HDX-MS and Cryo-EM significantly improves the understanding of structure–function relationships in complex protein systems, particularly those that are highly dynamic or difficult to crystallize.
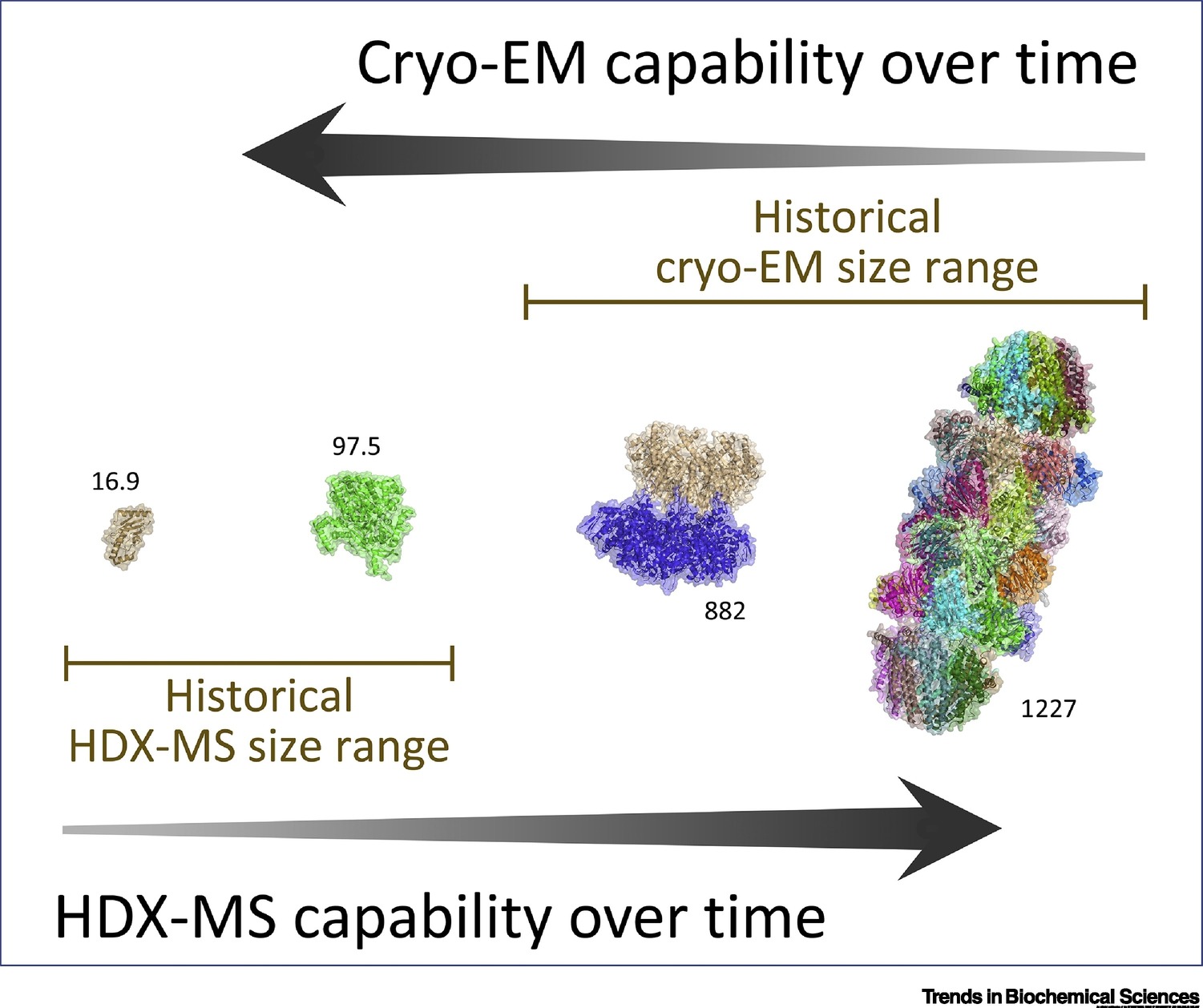
Engen, J R. et al. Trends in Biochemical Sciences, 2020.
Figure 2. HDX-MS Goes Larger While Cryo-EM Goes Smaller.
FAQ
Q1: How Does the Combined Application Improve Structural Research Accuracy?
A1: Cryo-EM provides in situ three-dimensional morphology, while mass spectrometry complements with information on protein composition, post-translational modifications, and interactions. Together, they enable integrated structure–function interpretation at nanometer-scale precision.
Q2: Are there Technical Compatibility Issues with the Combined Use?
A2: Sample preparation must meet the requirements of both techniques—for example, avoiding MS-interfering substances in buffer systems while maintaining structural stability. An experienced service team can provide optimization recommendations to ensure compatibility.
How to order?







