Collagen Lysine Hydroxylation Analysis Service
Collagen Lysine Hydroxylation Analysis Service is a specialized analytical service focused on identifying the site distribution, modification extent, and biological significance of lysine hydroxylation in collagen molecules. This service is broadly applicable to basic research, regenerative medicine, biomaterials development, studies of hereditary connective tissue disorders, and the mapping of tissue-specific modification profiles.
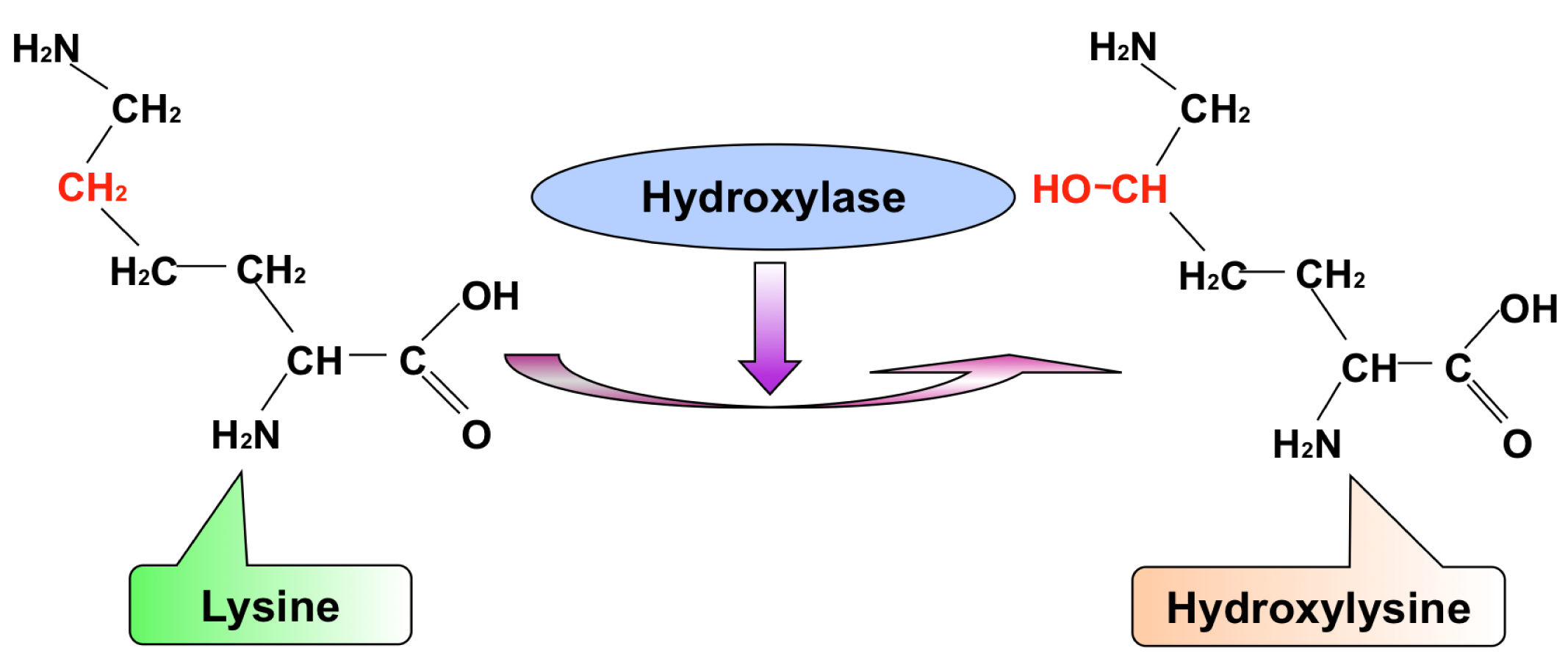
Xu Y. et al. Int J Mol Sci. 2014.
Collagen is the most abundant structural protein in vertebrates, widely distributed in tissues such as skin, bone, tendons, cartilage, and blood vessels. Its mechanical strength and scaffolding function are primarily supported by stable intra- and intermolecular cross-links, the formation of which critically depends on the hydroxylation of lysine (Lys) residues. Lysine hydroxylation is an enzyme-catalyzed modification mediated by the lysyl hydroxylase family (LH1–3), which targets specific sites within collagen molecules and converts Lys to hydroxylysine (Hyl). These Hyl residues can then participate in covalent cross-linking via aldehyde intermediates or undergo further glycosylation (e.g., glucose–galactose modifications), playing essential roles in regulating collagen structure, fibril assembly, and tissue remodeling. Dysregulation of lysine hydroxylation is closely associated with various diseases, including Ehlers-Danlos syndrome, osteogenesis imperfecta, fibrosis, cancer, and tissue degeneration. Therefore, accurate analysis of lysine hydroxylation in collagen is crucial for understanding tissue homeostasis, uncovering pathological mechanisms, and evaluating novel biomaterials and therapeutic approaches.
Using high-resolution mass spectrometry, nuclear magnetic resonance (NMR) structural analysis, and immunohistochemistry, MtoZ Biolabs provides Collagen Lysine Hydroxylation Analysis Service enabling comprehensive molecular, spatial, and structural characterization of lysine hydroxylation in collagen. This service precisely identifies modified sites, quantitatively evaluates modification levels, and elucidates the biological significance of lysine hydroxylation in tissue homeostasis, disease progression, and the functional regulation of biomaterials.
Analysis Workflow
The main workflow of Collagen Lysine Hydroxylation Analysis Service is as follows:
1. Sample Preparation
Receive samples and perform pretreatment and collagen extraction.
2. Enzymatic Digestion
Use trypsin or multi-enzyme strategies to digest collagen into detectable peptides.
3. Mass Spectrometry Detection
Collect mass spectrometry data of hydroxylysine-containing peptides using a high-resolution LC-MS/MS platform.
4. Data Analysis
Identify and annotate hydroxylysine modification sites, and evaluate their modification abundance and distribution patterns.
5. Structural/Tissue-Level Validation (Optional)
Use NMR spectroscopy and immunohistochemistry to assess structural changes and spatial distribution associated with the modifications.
6. Result Delivery
Provide modification maps, structural annotations, localization images, and a standardized quantitative report.
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs established an advanced Collagen Lysine Hydroxylation Analysis platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
3. High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all Collagen Lysine Hydroxylation Analysis data, providing clients with a comprehensive data report.
4. Compatibility with Complex Sample Types: Applicable to native tissues, engineered materials, and low-abundance recombinant samples.
Applications
Application Examples of Collagen Lysine Hydroxylation Analysis Service:
Mechanistic Studies of Connective Tissue Disorders
Reveal the role of aberrant hydroxylation in collagen structural defects and functional impairments.
Fibrosis and Cancer Research
Analyze changes in collagen modification patterns under different pathological conditions to understand mechanisms of extracellular matrix remodeling.
Tissue Engineering and Regenerative Medicine
Evaluate how collagen modification states in biomaterials affect tissue compatibility and mechanical properties.
Assessment of Orthopedic and Dental Materials
Monitor modification consistency in recombinant collagen products for quality control and structural optimization.
Functional Validation of Drug Targets
Assess the regulatory effects of lysyl hydroxylase inhibitors or related modification enzyme inhibitors on target protein modification levels.
FAQ
Q. Can your Service Identify All Types of Collagen?
Yes. MtoZ Biolabs supports hydroxylation analysis of major collagen types including type I, II, and III, whether derived from native tissues, extracellular matrix extracts, or recombinant expression products. For special collagen types (e.g., type IV, type X), please notify us in advance so we can optimize the digestion protocol and database annotation parameters.
Q. Can you Precisely Localize the Specific Lysine Hydroxylation Sites?
Yes. Using high-resolution mass spectrometry, MtoZ Biolabs can accurately identify lysine residues modified by hydroxylation at the peptide level and provide corresponding MS spectra and site-specific annotation details.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
Post-Translational Modifications Proteomics Service
Quantitative Phosphoproteomics Service
Quantitative Glycoproteomics Service
How to order?







