Co Immunoprecipitation (Co-IP) Service
- The target protein is captured in its native state, minimizing the potential for experimental artifacts.
- Complete protein complexes can be isolated, making it a valuable tool for studying protein functions and their pathways.
- Co-IP is compatible with a variety of sample preparation and protein identification techniques.
- The method is simple and straightforward, making it easy to implement.
- Cutting-edge Technology: Our use of advanced equipment like the Thermo Fisher Orbitrap Fusion Lumos mass spectrometer ensures high sensitivity and resolution for protein identification.
- Expert Team: Our experienced team has extensive knowledge in Co-IP and mass spectrometry, providing specialized support for your research.
- Customized Solutions: We offer flexible experimental plans tailored to your research needs, ensuring precise and reliable results.
- One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Co immunoprecipitation (Co-IP) is a widely established technique for studying protein-protein interactions under native conditions in vivo. By using specific antibodies to capture target proteins, co immunoprecipitation (Co-IP) enables the isolation of complex chaperone proteins, structural proteins, cofactors, and signaling molecules, shedding light on their essential roles in cellular processes. This technique allows researchers to identify both direct and indirect interactions between proteins, offering insights into how proteins collaborate within cells to drive complex biological mechanisms. Coupling co immunoprecipitation (Co-IP) with mass spectrometry not only improves the efficiency of detecting protein-protein interactions but also provides accurate quantitative data and detailed information on protein modifications.
MtoZ Biolabs offers high-quality Co Immunoprecipitation (Co-IP) Service with the Thermo Fisher Orbitrap Fusion Lumos mass spectrometer and Nano-LC technology to support researchers in exploring the complex relationships between proteins.
Technical Principles
Co immunoprecipitation (Co-IP) works by capturing target proteins with specific antibodies, followed by the identification of interacting proteins through subsequent steps. The process begins by mixing cell lysates or other biological samples with the target antibody to form an antibody-antigen complex. Magnetic beads or other solid-phase supports are then used to isolate the complex. After washing to eliminate non-specifically bound proteins, the interacting partners are identified using Western blotting or mass spectrometry.
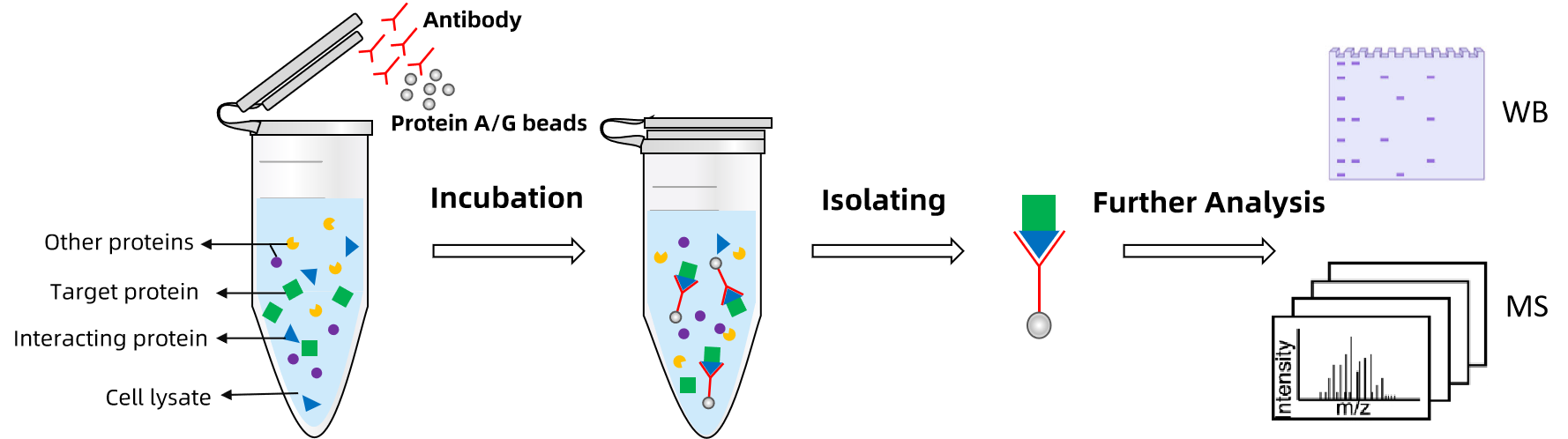
Technical Advantages
Compared to other protein interaction methods, co immunoprecipitation (Co-IP) offers the following advantages:
Analysis Workflow
1. Sample Preparation
Proteins are extracted from cells or tissues to prepare cell lysates.
2. Immunoprecipitation
Specific antibodies are added to bind the target protein and form an antibody-protein complex.
3. Washing
Multiple washes remove non-specifically bound components, enriching the target protein and its interacting partners.
4. Mass Spectrometry
The precipitated proteins are analyzed using mass spectrometry.
5. Data Analysis
Specialized software is used to analyze the mass spectrometry data, identifying and quantifying protein interactions.
Why Choose MtoZ Biolabs?
MtoZ Biolabs is equipped with an advanced mass spectrometry platform and a dedicated team of experts committed to providing efficient and reliable Co Immunoprecipitation (Co-IP) Service. We offer:
Applications
• Signal Transduction: Study interactions between key proteins in signaling pathways.
• Protein Complex Identification: Investigate the composition and functions of specific protein complexes.
• Disease Mechanisms: Analyze changes in protein interactions in disease
Case Study
Case 1: Analysis of protein interactions between Eimeria maxima sporozoites and chicken jejunal epithelial cells using co immunoprecipitation (Co-IP) combined with LC-MS/MS.
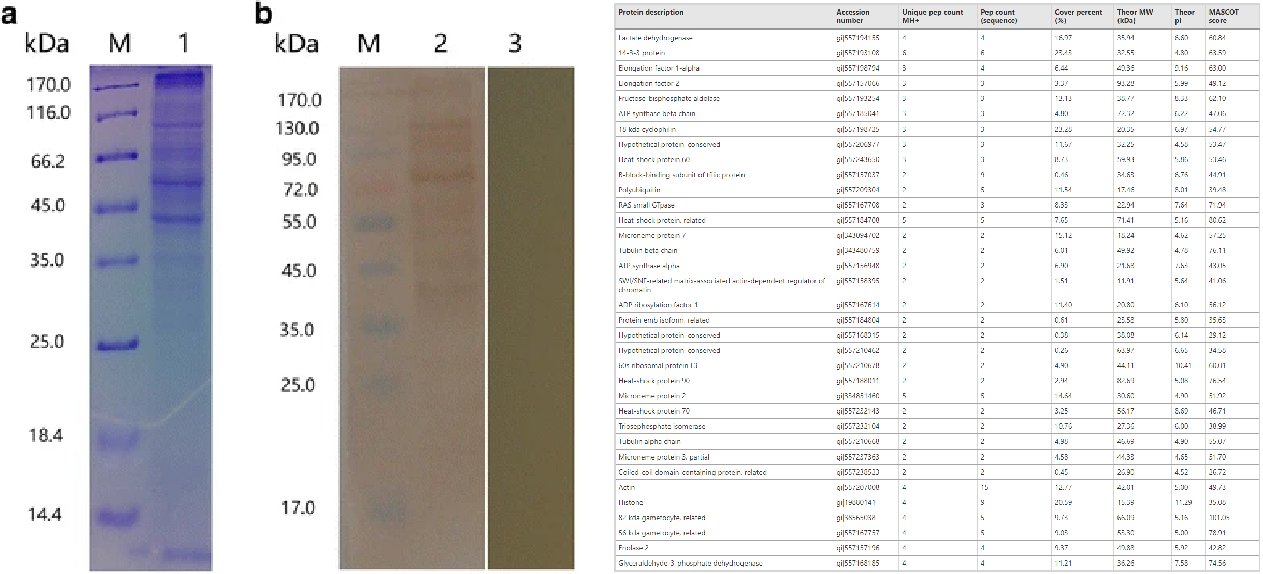
Huang, J. W. et al. Parasites Vectors, 2018.
Case 2: Investigation of the interaction between TRIM28 and nuclear receptors in regulating uterine function using co immunoprecipitation (Co-IP).
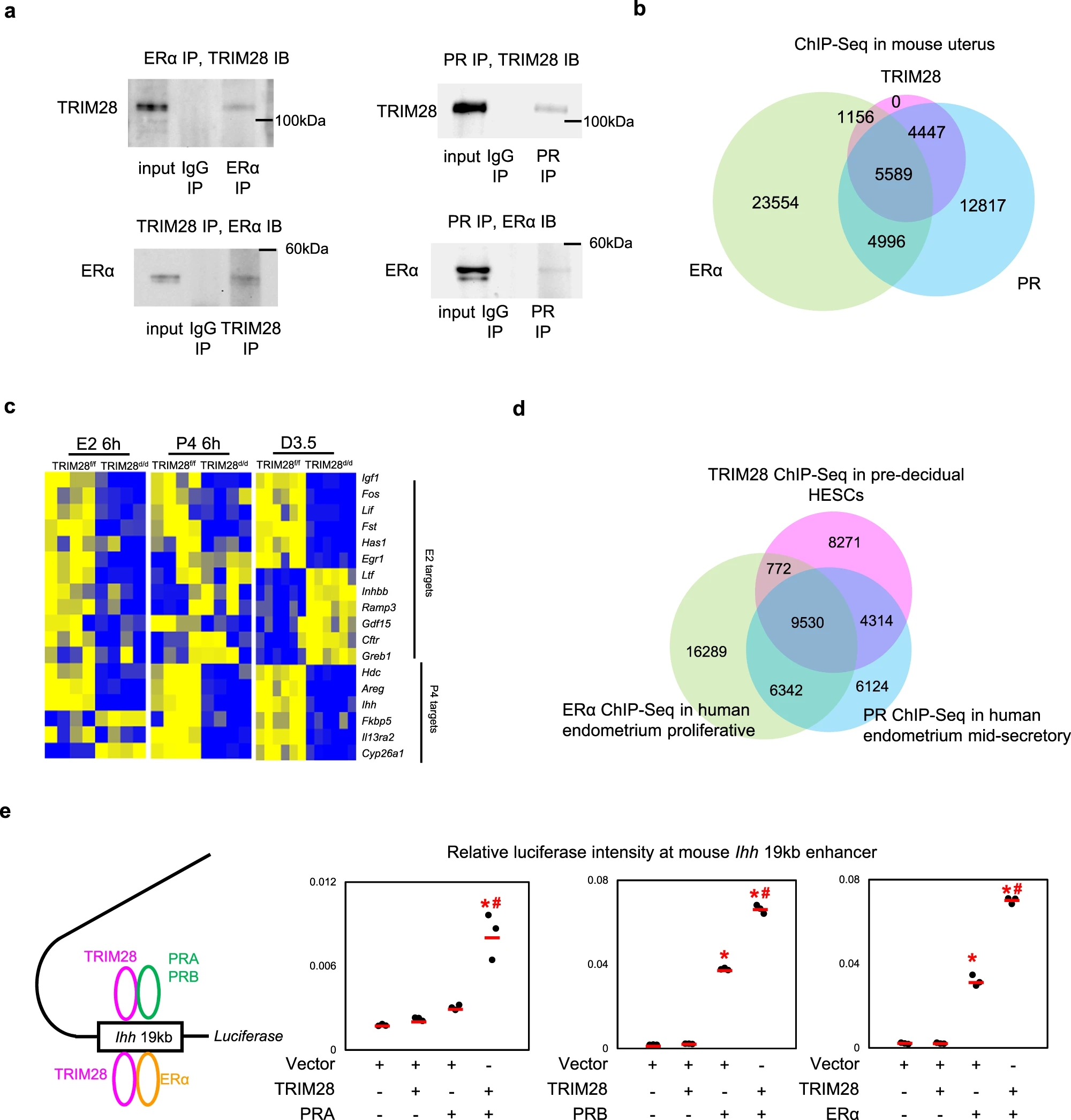
Li, R. et al. Nat. Commun. 2023.
FAQ
Q1: What is the difference between IP and Co-IP?
Immunoprecipitation (IP) purifies specific proteins by binding an antigen to an antibody. Co Immunoprecipitation (Co-IP) is an extension of IP that focuses on studying protein-protein interactions within specific complexes. Co-IP is aimed at identifying interactions between proteins, rather than secondary antibody-protein interactions.
Q2: What are key considerations during sample preparation?
It is important to use an appropriate cell lysis buffer to maintain protein integrity, ensure protein concentration is 1-5 mg/mL, and use fresh samples to prevent degradation. Selecting the right antibody is also crucial to improving the precipitation efficiency.
Q3: How to select tags for co immunoprecipitation (Co-IP) experiments?
Tags should be chosen based on the target protein's expression level, stability, and binding sites. Common tags like HA, Myc, and FLAG offer good specificity and affinity for efficient immunoprecipitation without interfering with protein function.
MtoZ Biolabs offers expert Co Immunoprecipitation (Co-IP) Service to support your protein-protein interaction studies. Whether for basic research, disease mechanism exploration, or drug development, we provide reliable technical support for your research. Contact us for more information.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Protein-Protein Interaction Analysis Service
SILAC Based Co-IP-MS for Protein Interaction Analysis Service
How to order?







