Cellulose Acetate Analysis Service | Pharmaceutical Excipient
Cellulose acetate (CA) is a pharmaceutical excipient derived from the acetylation of natural cellulose, typically appearing as a white or off-white powder with good film-forming ability, mechanical strength, and biocompatibility. In pharmaceutical formulations, it is commonly used as a film former, matrix material, or controlled-release carrier, enabling the regulation of drug release rates and enhancing formulation stability and bioavailability. The analysis of cellulose acetate is usually based on physicochemical testing and structural characterization methods to evaluate its degree of acetylation, molecular structure, purity, and thermal stability.
Cellulose acetate analysis service based on pharmaceutical excipients has broad applications in the pharmaceutical field. It can be used for quality control and formulation optimization of carrier materials in controlled-release and sustained-release formulations, ensuring consistency and stability across different production batches. At the same time, in the development of biopharmaceuticals and oral solid dosage forms, CA analysis helps assess the compatibility of excipients with active ingredients. In addition, this service is also applied in the research and development of food, nutritional supplements, and other health products, providing scientific data support for product safety, stability, and functionality.
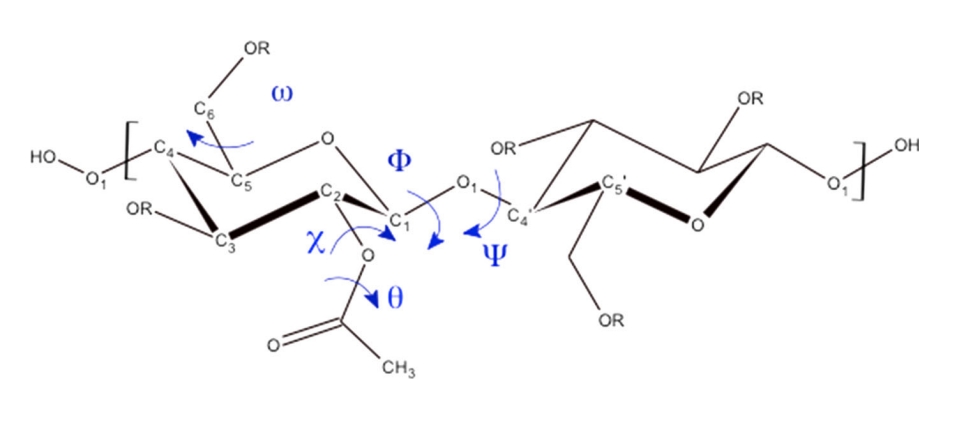
Bocahut, A. et al. Cellulose, 2014.
Figure 1. Schematic Representation of a Dimer in a CA Chain.
Services at MtoZ Biolabs
Based on advanced analytical platforms, MtoZ Biolabs has launched the cellulose acetate analysis service based on a pharmaceutical excipient, which enables comprehensive detection of the key characteristics of cellulose acetate. This service relies on a variety of advanced instruments to accurately evaluate its chemical composition, physical properties, thermal performance, and solubility. Through systematic analysis, researchers can obtain data on the structural characteristics, physicochemical properties, and stability of cellulose acetate under different conditions, providing reliable support for pharmaceutical formulation development, excipient quality control, and formulation optimization. MtoZ Biolabs offers services including, but not limited to, the following:
1. Chemical Composition
High-performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) are used to analyze the chemical composition and degree of acetylation of cellulose acetate, ensuring accurate composition and clear structural characteristics.
2. Physical Properties
Scanning electron microscopy (SEM) and particle size analysis techniques are employed to evaluate the morphology, particle size distribution, and surface structure of samples, combined with mechanical testing to characterize physical performance.
3. Thermal Performance
Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) are applied to assess the thermal stability and decomposition behavior of cellulose acetate, revealing its performance under different processing and storage conditions.
4. Solubility
Ultraviolet-visible spectroscopy (UV-Vis) and viscosity measurement methods are used to characterize its solubility and rheological properties in different solvents, evaluating its applicability in practical pharmaceutical formulations.
Sample Submission Suggestions
1. Sample Type
Applicable to pharmaceutical excipient samples containing cellulose acetate (CA), including powder, granule, or solution forms. The source of the sample should be clearly identified and representative.
2. Sample Purity
It is recommended to remove impurities or non-target components as much as possible to avoid interference with analytical results and to improve the accuracy of composition, physical properties, and thermal stability testing.
3. Sample Storage and Transportation
Samples should be stored under dry and light-protected conditions to prevent moisture absorption and degradation. During transportation, sealed packaging is recommended, and low temperature or ice packs can be used if necessary to maintain sample stability.
Service Advantages
1. High-Precision Analysis
Relying on advanced platforms such as HPLC, MS, and NMR, cellulose acetate’s chemical composition and structural characteristics can be accurately analyzed to ensure reliable data.
2. Multidimensional Characterization
By combining tests of physical properties, thermal performance, and solubility, cellulose acetate’s overall performance can be comprehensively evaluated, providing systematic support for pharmaceutical excipient development.
3. Customized Solutions
Analysis strategies are tailored according to sample characteristics and research objectives, flexibly meeting the needs of both R&D and quality control.
4. One-Stop Service
Covering sample preparation, analytical testing, data interpretation, and report generation, a complete solution is provided to simplify workflows and improve efficiency.
Applications
1. Pharmaceutical Formulation Development
By analyzing the solubility, thermal performance, and physical properties of cellulose acetate, its suitability in controlled-release, sustained-release, and film-coating formulations can be evaluated to optimize drug release performance.
2. Quality Control
Cellulose acetate analysis service can be used to detect the purity, chemical composition, and impurity levels of cellulose acetate, ensuring consistency and compliance in pharmaceutical production processes.
3. Formulation Optimization Research
By analyzing the characteristics of cellulose acetate under different conditions, reliable data support can be provided for formulation improvement and process optimization.
4. Excipient Performance Evaluation
Cellulose acetate analysis service can be applied to systematically study its rheological properties and solution behavior, verifying its performance as a binder, film-forming agent, or stabilizer, and supporting the rational application of pharmaceutical excipients.
FAQ
Q1: Can Low-Purity or Complex Samples Be Tested?
A1: Yes. Through sample pretreatment and purification, we can remove most impurities to ensure the accuracy and reproducibility of the final test results.
Q2: What Does the Results Report Include?
A2: The report usually includes the chemical composition, purity, impurity information, thermal stability, and solubility characteristics of cellulose acetate, as well as its functional performance under specific conditions, ensuring that researchers receive comprehensive data support.
How to order?







