CD Spectroscopy vs FTIR: Which Technique is Better for Analyzing Protein Secondary Structure?
The structural organization of a protein dictates its biological function, with secondary structure representing a pivotal level in this hierarchy. Alterations in α-helices, β-sheets, and random coils are frequently associated with functional transitions of proteins, including partial or complete inactivation. Consequently, accurate characterization of protein secondary structure is essential not only for fundamental research but also for applications such as drug development, evaluation of biopharmaceutical stability, and studies of structure–function relationships. Currently, circular dichroism (CD) spectroscopy and Fourier-transform infrared (FTIR) spectroscopy are the two most widely employed spectroscopic techniques for secondary structure analysis. Each method presents distinct advantages and inherent limitations. Which, then, is better suited to your research objectives?
CD Spectroscopy: Rapid and Sensitive, but Dependent on Sample Purity
1. Principle and Advantages
CD spectroscopy exploits the interaction of circularly polarized light with chiral molecular structures to probe protein conformation. Characteristic CD signals corresponding to α-helices, β-sheets, and random coils are observed within the 190–250 nm range. By fitting these spectra to curated reference libraries, the relative proportions of secondary structural elements can be rapidly estimated. CD spectroscopy is particularly advantageous for:
(1) Real-time monitoring of protein conformational changes under varying pH, temperature, or solvent conditions
(2) Determining the secondary structure distribution of proteins with a single dominant conformation
(3) Performing measurements at relatively low protein concentrations (typically 0.1–1 mg/mL)
2. Limitations
Despite its sensitivity, CD spectroscopy requires high sample purity and is highly susceptible to interference from impurities, buffer absorbance, and non-protein background signals. Furthermore, spectral interpretation relies on curve-fitting algorithms based on reference spectral databases, which reduces accuracy when analyzing heterogeneous conformations or complex protein mixtures.
FTIR: Robust and Versatile, but with Limited Structural Resolution
1. Principle and Advantages
FTIR spectroscopy characterizes protein secondary structure by detecting molecular vibrational modes, most notably the amide I band (approximately 1600–1700 cm⁻¹). This spectral region is particularly sensitive to the vibrations of α-helices, β-sheets, and random coils. The key benefits of FTIR include:
(1) Compatibility with solid, liquid, and lyophilized protein samples
(2) Relative insensitivity to buffer composition, enabling use with complex matrices
(3) Suitability for high-concentration samples, including those in pharmaceutical formulations
(4) Capability for thermal stability assessments when coupled with temperature control
2. Limitations
Although broadly applicable, FTIR generally provides lower quantitative precision for secondary structure analysis, particularly in spectral regions where multiple component peaks overlap, limiting structural resolution. Additionally, water absorption in the amide I region necessitates correction techniques, such as difference spectroscopy, to remove background interference.
CD Spectroscopy vs FTIR: How Should You Choose?
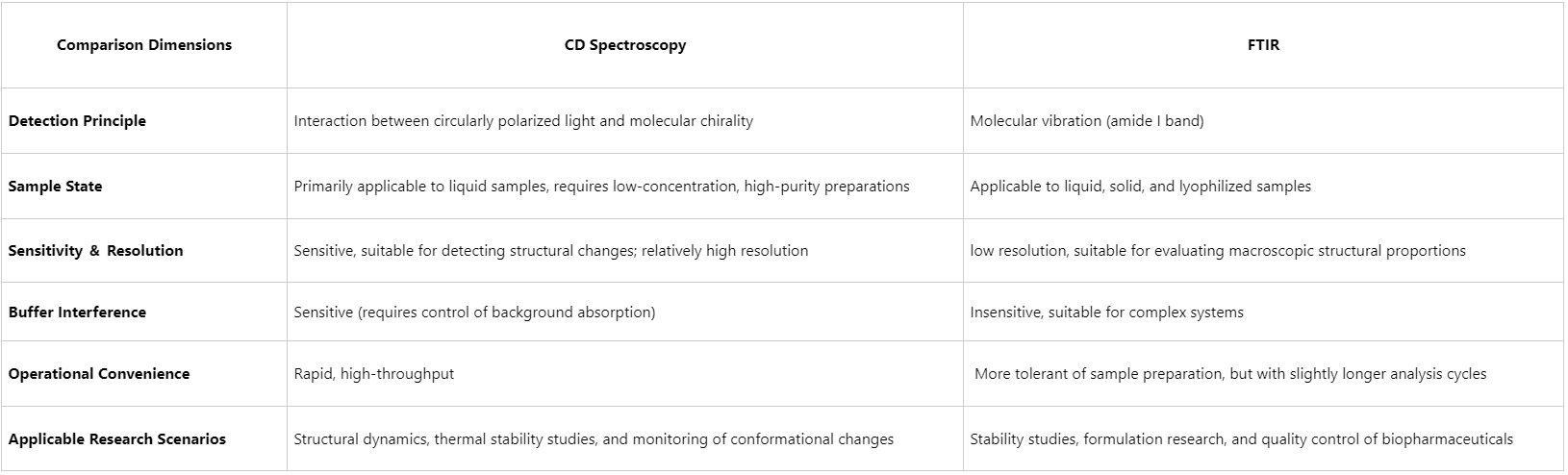
Figure 1. CD Spectroscopy vs FTIR
Practical Recommendations: A Goal-Oriented or Combined Approach
In protein secondary structure analysis, there is no universally “best” technique, only the “most appropriate” one for a given objective. For rapid screening of conformational dynamics, CD spectroscopy is typically preferred; for evaluating structural stability within complex environments or at high protein concentrations, FTIR provides a distinct advantage. Importantly, these methods are complementary rather than mutually exclusive. Many studies employ a combined CD–FTIR strategy to achieve more comprehensive and accurate structural insights.
Every step in the workflow — from experimental design and sample handling to spectral analysis — critically impacts data quality. MtoZ Biolabs leveraging advanced mass spectrometry platforms, offers professional CD spectroscopy services for proteins. Our technical team tailors experimental protocols to meet specific research goals, facilitating the efficient progression of both scientific investigation and product development.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







