Carboxymethylcellulose Sodium Analysis Service | Pharmaceutical Excipient
- Sample Reception and Registration: SSG samples are received, labeled, and recorded with batch information.
- Pre-treatment: Samples are prepared based on test requirements, including drying, sieving, or dissolution.
- Physical Property Analysis: Assessment of appearance, particle size, flowability, and viscosity.
- Chemical and Structural Analysis: Determination of DS, solubility, chloride/sodium content, and residual monochloroacetic acid using FTIR, NMR, HPLC, or GC.
- Functional Testing: Evaluation of thickening, gelation, and binding effects in pharmaceutical formulations.
- Safety and Impurity Assessment: Analysis of heavy metals, microbial contamination, and moisture content.
- Data Compilation and Report Generation: All results are compiled into a comprehensive report with conclusions and recommendations.
- Sample Quantity: At least 5–10 g of CMC-Na powder per analysis.
- Packaging: Sealed, dry, and contamination-free containers.
- Documentation: Batch number, manufacturer, production date, and SDS (Safety Data Sheet) if available.
- Shipping Conditions: Protect from high temperature and moisture; standard courier or refrigerated shipping is acceptable.
- Comprehensive analytical report covering physical, chemical, functional, and safety evaluation results.
- Instrument data and raw measurement files (upon request).
- Conclusions and recommendations to support R&D, formulation, and quality control decisions.
Carboxymethylcellulose sodium (CMC-Na) is a water-soluble cellulose derivative widely used as a pharmaceutical excipient in solid and semi-solid dosage forms. It is synthesized by chemically modifying natural cellulose with monochloroacetic acid under alkaline conditions, resulting in the introduction of carboxymethyl groups. This modification enhances the solubility, viscosity, and stabilizing properties of cellulose, making CMC-Na a versatile excipient for tablets, capsules, suspensions, and gels. In addition to functioning as a binder, thickening agent, and stabilizer, CMC-Na can improve the uniformity, mechanical strength, and release profile of pharmaceutical formulations.
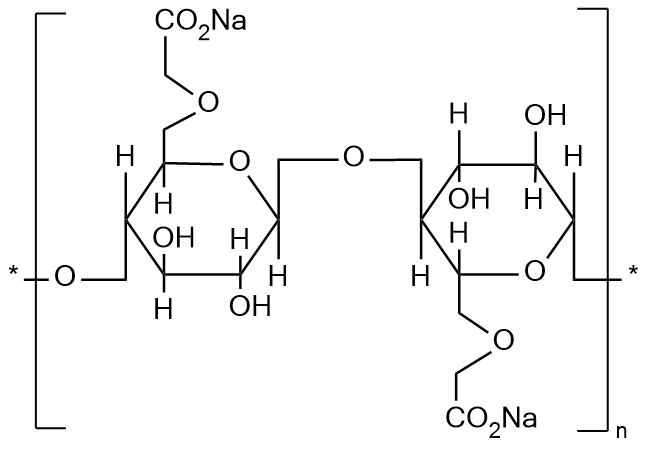
Figure 1. The Structure of Carboxymethylcellulose Sodium
As a professional provider of pharmaceutical excipient analysis services, MtoZ Biolabs offers a comprehensive carboxymethylcellulose sodium analysis service by systematically evaluating physical properties, chemical composition, functional performance, and safety to ensure CMC-Na meets international pharmacopeia standards and supports pharmaceutical R&D and manufacturing quality control.
Services at MtoZ Biolabs
MtoZ Biolabs’ carboxymethylcellulose sodium analysis service covers a wide range of analyses, including physical, chemical, functional, and safety evaluations. Detailed analytical categories are summarized in the table below:
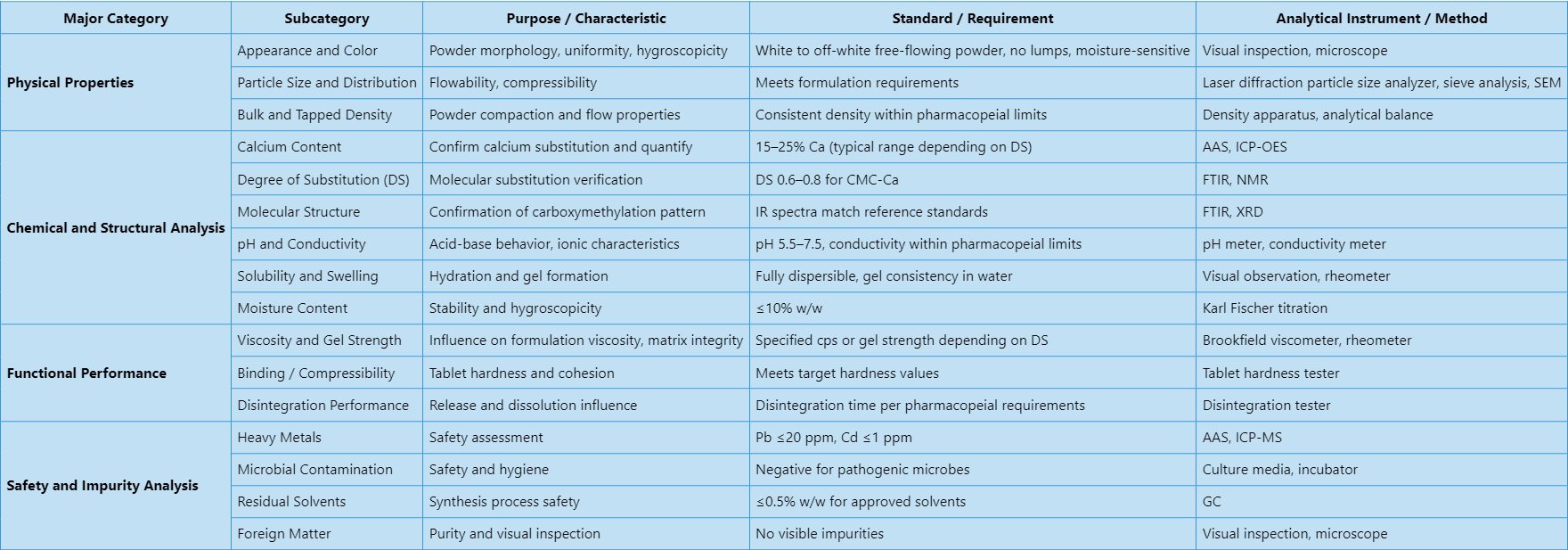
Analysis Workflow
Service Advantages
1. Comprehensive Analysis Coverage
MtoZ Biolabs’ carboxymethylcellulose sodium analysis service evaluates CMC-Na across physical, chemical, functional, and safety parameters, providing a holistic understanding of excipient quality to support formulation development.
2. Strict Compliance with Pharmacopeia Standards
All analyses adhere to USP, EP, and other international pharmacopeia standards, ensuring results meet global regulatory and quality requirements for pharmaceutical excipients.
3. Advanced Instrumentation Support
Our laboratory is equipped with FTIR, NMR, HPLC, GC, rheometer, viscometer, AAS, ICP-MS, and particle size analyzers to ensure precise, accurate, and reproducible results for all analyses.
4. Efficient and Timely Execution
Optimized workflow allows for rapid completion of standard and customized analyses, enabling timely support for R&D, quality control, and production operations.
5. Safety and Regulatory Assurance
Monitoring of heavy metals, residual reagents, moisture, and microbial contamination ensures that CMC-Na meets safety requirements, providing confidence for formulation design and regulatory submissions.
Applications
✔️Solid Dosage Form Development: Evaluation of CMC-Na as a binder, thickener, or stabilizer in tablets and capsules.
✔️Pharmaceutical Quality Control: Raw excipient verification before production or formulation.
✔️Process Optimization: Assessment of functional properties under various formulation or processing conditions.
✔️Excipient Innovation: Supporting the development of modified cellulose derivatives or new excipient sources.
FAQs
Q1: What Is the Standard for CMC-Na Viscosity Testing?
A1: Viscosity is typically measured at 1% aqueous solution and should meet pharmacopeial specifications or specific formulation requirements.
Q2: Why Is Residual Monochloroacetic Acid Tested?
A2: Residual monochloroacetic acid is a by-product of CMC-Na synthesis. Testing ensures safety and compliance with regulatory limits (≤0.05%).
Q3: How Long Does the Carboxymethylcellulose Sodium Analysis Service Take?
A3: Standard analysis is typically completed within 7–10 working days, depending on the combination of tests.
Sample Submission Suggestions
Deliverables
MtoZ Biolabs provides professional carboxymethylcellulose sodium analysis service, offering full-spectrum analysis from physical and chemical properties to functional performance and safety. Whether for excipient screening during R&D or quality control in production, our service ensures reliable, accurate, and regulatory-compliant data support.
Contact us today to submit your CMC-Na samples and receive a detailed, authoritative analysis report to ensure excipient quality, safety, and compliance.
How to order?







