Calcium Saccharate Analysis Service | Pharmaceutical Excipient
Calcium saccharate is a common pharmaceutical excipient, an organic complex formed by gluconic acid and calcium salt, with good stability and biocompatibility. It is often used in pharmaceutical formulations as a sustained-release material, stabilizer, or excipient to improve the physicochemical stability and bioavailability of drugs. Calcium saccharate is particularly common in solid dosage forms, and its quality and purity have a direct impact on the safety and effectiveness of drugs. In recent years, regulatory authorities have imposed increasingly strict requirements on the quality control of excipients, so systematic and scientific analysis and testing of calcium saccharate has become a key step in drug development and production.
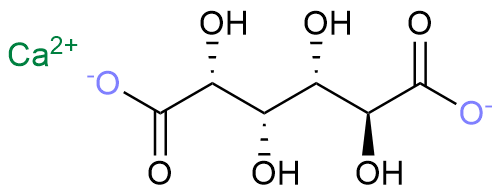
Figure 1. The Structure of Calcium Saccharate
MtoZ Biolabs offers advanced pharmaceutical excipient analysis services, including pharmaceutical impurity, drug microbial limit, drug composition, API analysis and drug structure confirmation service, to clients such as excipient manufacturers, biopharmaceutical companies, and academic institutions. Building on these broad analytical capabilities, we also provide specialized calcium saccharate analysis service that supports comprehensive quality evaluation from raw material control to formulation application, ensuring compliance with pharmacopeia standards and delivering reliable data for drug development and production.
Services at MtoZ Biolabs
Our calcium saccharate analysis service includes:
|
🔸 Content Determination Using inductively coupled plasma mass spectrometry (ICP-MS) or atomic absorption spectroscopy (AAS) to accurately quantify calcium ion content, combined with high-performance liquid chromatography (HPLC) or ion chromatography to detect gluconate, ensuring the proper proportion of excipient components. |
🔸 Impurity and Residual Analysis Systematically evaluating metal impurities, organic impurities, and residual solvents in samples to ensure excipient purity meets pharmacopeia standards and reduce potential impact on formulation quality and safety. |
🔸 Structural Identification and Feature Confirmation Using nuclear magnetic resonance (NMR), Fourier-transform infrared spectroscopy (FTIR), and mass spectrometry (MS) to confirm the molecular structure and binding state of calcium saccharate, verifying its consistency and source reliability. |
|
🔸 Physicochemical Property Testing Including routine physicochemical parameter testing such as moisture content, pH value, solubility, and particle size distribution, providing reference for formulation development. |
🔸 Stability and Compatibility Studies Evaluating the stability of calcium saccharate under different environmental conditions, while testing its compatibility with APIs and other excipients to reduce process risks. |
🔸 Method Development and Validation Developing and validating dedicated analytical methods based on customer needs to ensure specificity, sensitivity, precision, and repeatability, meeting registration and compliance requirements. |
|
🔸 Quality Control Support Providing pharmacopeia-compliant testing services and comparative analysis to help clients achieve full-process quality control in raw material control, production, and product release. |
Analysis Workflow
1. Sample receipt and pretreatment: confirm basic information and perform dissolution, dilution, or filtration according to experimental requirements.
2. Component and impurity testing: complete quantitative determination of calcium ions and gluconate and impurity measurement using multi-platform instruments.
3. Structural and property analysis: confirm structure and binding state through spectroscopic and mass spectrometric methods.
4. Data processing and result integration: combine raw data, reference materials, and statistical analysis to obtain comprehensive conclusions.
5. Report delivery: generate a complete analysis report including charts, results, and method descriptions.
Service Advantages
1. Advanced Platforms
MtoZ Biolabs is equipped with multiple high-resolution analytical platforms, covering mass spectrometry, spectroscopy, and chromatography, capable of providing reliable testing solutions for the complex structural characteristics of calcium saccharate.
2. Reliable Data Quality
Our calcium saccharate analysis service strictly follows pharmacopeia and industry standards to ensure precision and repeatability in testing.
3. Comprehensive Service Content
From content determination to impurity evaluation, and stability and performance testing, MtoZ Biolabs provides a one-stop calcium saccharate testing solution. Clients can flexibly select testing items according to their R&D or production needs to improve efficiency.
4. Professional Team Support
Our scientific team has many years of experience in pharmaceutical excipient analysis, familiar with common analytical methods and application scenarios, and can provide clients with scientifically reasonable testing strategies and technical support to help reduce risks in R&D and quality control.
5. Customizable Services
MtoZ Biolabs provides flexible testing programs based on the specific needs of client projects.
Applications
1️⃣ Drug development and production: control excipient quality to ensure stability and consistency of formulations.
2️⃣ Quality control and compliance testing: meet pharmacopeia and regulatory requirements, support registration submissions.
3️⃣ Formulation process optimization: analyze excipient properties to guide drug release and dissolution studies.
4️⃣ Excipient substitution and comparative studies: provide comparative data for excipients of different sources or production processes.
FAQs
Q1: How long does the calcium saccharate analysis service take?
A1: In general, the standard analysis cycle is 3–6 weeks, depending on the number of samples and analysis items.
Sample Submission Suggestions
1. Sample type: solid or solution, preferably raw material or intermediate.
2. Sample quantity: ≥50 mg or 1 mL for routine testing; special projects need further confirmation.
3. Storage conditions: recommended low temperature, protected from light, sealed; use dry ice or ice packs during transportation.
4. Information required: clients should provide basic sample information, such as batch number, source, and intended use.
Deliverables
1. PDF report with detailed experimental results
2. Raw data files and corresponding spectra
3. Statistical analysis and result interpretation
4. Methodological parameters and QC results when necessary
Calcium saccharate, as a key pharmaceutical excipient, directly affects the safety and effectiveness of drugs. With a complete analytical platform and a professional team, MtoZ Biolabs provides systematic and reliable calcium saccharate analysis service that supports comprehensive evaluation of excipient quality. If you are looking for a professional excipient testing partner, please contact us. We will safeguard your R&D and production and help your projects progress smoothly.
How to order?







