Bacterial Glycosylation Analysis Service
Bacterial Glycosylation Analysis Service refers to a service that utilizes high-sensitivity mass spectrometry in combination with glycopeptide enrichment and other multidimensional analytical techniques to systematically identify, structurally characterize, and quantitatively compare bacterial glycoproteins and their glycosylation types, sites, glycan structures, and functional implications. This service is well-suited for investigating the structural and functional regulation of bacterial glycosylation in pathogenicity, host interaction, immune evasion, vaccine target discovery, and natural product glycan modification. It offers technical advantages for analyzing bacterial samples with high glycan heterogeneity, extensive structural diversity, and limited database coverage.
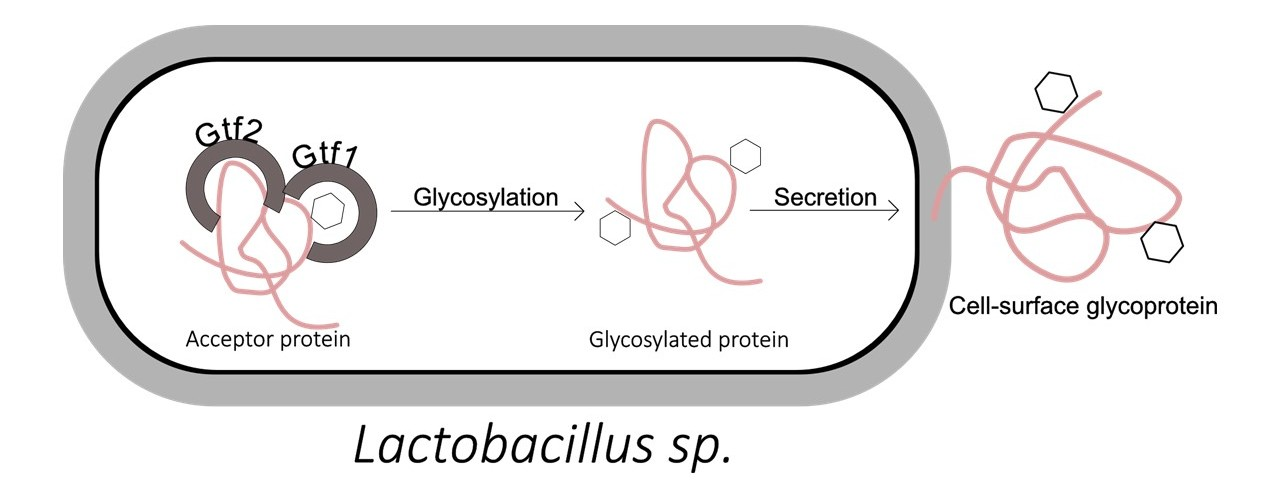
Glycosylation is an important post-translational modification widely present in eukaryotic cells. Recent studies have revealed that bacteria also possess complex glycosylation systems, which include not only N-linked and O-linked glycosylation, but also a range of novel glycan structures and atypical enzymatic pathways. Bacterial glycosylation plays critical roles in regulating pathogenicity, immune evasion, host recognition, biofilm formation, vaccine design, and microbial ecological adaptability.
Latousakis D, Juge N. Int J Mol Sci. 2018.
Unlike the highly conserved nature of eukaryotic glycosylation, bacterial glycosylation exhibits greater structural diversity, enzymatic pathway variability, and glycan heterogeneity. As a result, its analysis presents higher technical challenges and imposes stricter requirements on glycan structure elucidation, modification site identification, and species-specific annotation. With advances in high-resolution mass spectrometry and glycoprotein enrichment technologies, bacterial glycosylation analysis has become an essential component of microbiome research, infection biology, and biopharmaceutical development.
Leveraging high-resolution mass spectrometry and other platforms, MtoZ Biolabs offers Bacterial Glycosylation Analysis Service that can identify N-sugar and O-sugar modification sites in bacterial proteins with high sensitivity, analyze sugar chain structures and isomeric forms, support quantitative comparison and functional annotation of multiple samples, and help customers deeply reveal the key role of glycosylation in bacterial pathogenicity, immune regulation and vaccine development.
Analysis Workflow
The main workflow of Bacterial Glycosylation Analysis Service is as follows:
1. Sample Preparation
Total proteins are extracted from bacterial cultures and subjected to standardized quantification.
2. Proteolytic Digestion
Proteins are digested into detectable peptides using enzymes such as trypsin.
3. Glycopeptide or Glycoprotein Enrichment
Glycosylated peptides are enriched using HILIC, lectin affinity chromatography, or hydrazide-based capture methods.
4. High-Resolution Mass Spectrometry Analysis
LC-MS/MS is used to acquire MS1 and MS2 spectra of glycopeptides, obtaining both glycan and peptide information.
5. Data Analysis and Glycan Annotation
Glycopeptide identification software is used to annotate modification sites and glycan structures, which are then compiled into a comprehensive report.
Applications
Example applications of Bacterial Glycosylation Analysis Service include:
Glycosylation Analysis of Pathogen Virulence Factors
Identify glycosylation on key factors such as outer membrane proteins and toxins, and assess their impact on bacterial pathogenicity.
Glycan Profiling of Vaccine Antigens
Characterize how glycosylation affects antigen recognition, T/B cell responses, and vaccine immunogenicity.
Host-Pathogen Interaction Mechanism Studies
Elucidate adhesion, invasion, and immune modulation mechanisms mediated by glycosylation.
Coupling of Glycosylation and Secretion Systems
Explore regulatory roles of glycosylation in type III/IV bacterial secretion systems.
Glycosyltransferase Function and Target Screening
Systematically investigate substrate specificity and regulatory networks of glycosyltransferases such as PglB and O-oligosaccharyltransferase.
FAQ
Q. Does this Service Support Identification of Noncanonical Glycan Structures Unique to Bacteria?
Yes. We support the identification of atypical monosaccharide structures commonly found in bacteria. By applying open modification search strategies, custom glycan mass libraries, and high-resolution MS/MS (including HCD and ETD/EThcD fragmentation modes), we can accurately detect unique glycan mass features and confirm linkage types and modification sites through manual spectral validation. This allows for adaptation to a broad range of bacterial species and unknown glycan types.
Q. Can Glycosylation Sites be Accurately Localized, Especially in the Presence of Multiple Glycan Chains or Glycopeptide Isomers?
Yes. We apply multi-mode fragmentation strategies (HCD + EThcD) to preserve glycan integrity while ensuring complete peptide backbone fragmentation. Combined with glycosite localization scoring models, this enables accurate site localization even in the presence of isomeric glycopeptides. For multi-glycan modifications or linkage isomers (e.g., O-glycosylation at different positions), we also offer manual spectral review and isomer screening services to ensure reliable, publication-quality site resolution.
Case Study
Using high-resolution mass spectrometry and glycopeptide enrichment strategies, this study confirmed that C. hepaticus HV10T is capable of adding N-linked heptasaccharides to more than 35 proteins, validating the presence of a functional N-glycosylation system. Comparative analysis further revealed that this strain shares several conserved glycoproteins associated with host colonization with C. jejuni, while also possessing unique glycosylated proteins that may contribute to its adaptation and pathogenicity within the host environment.
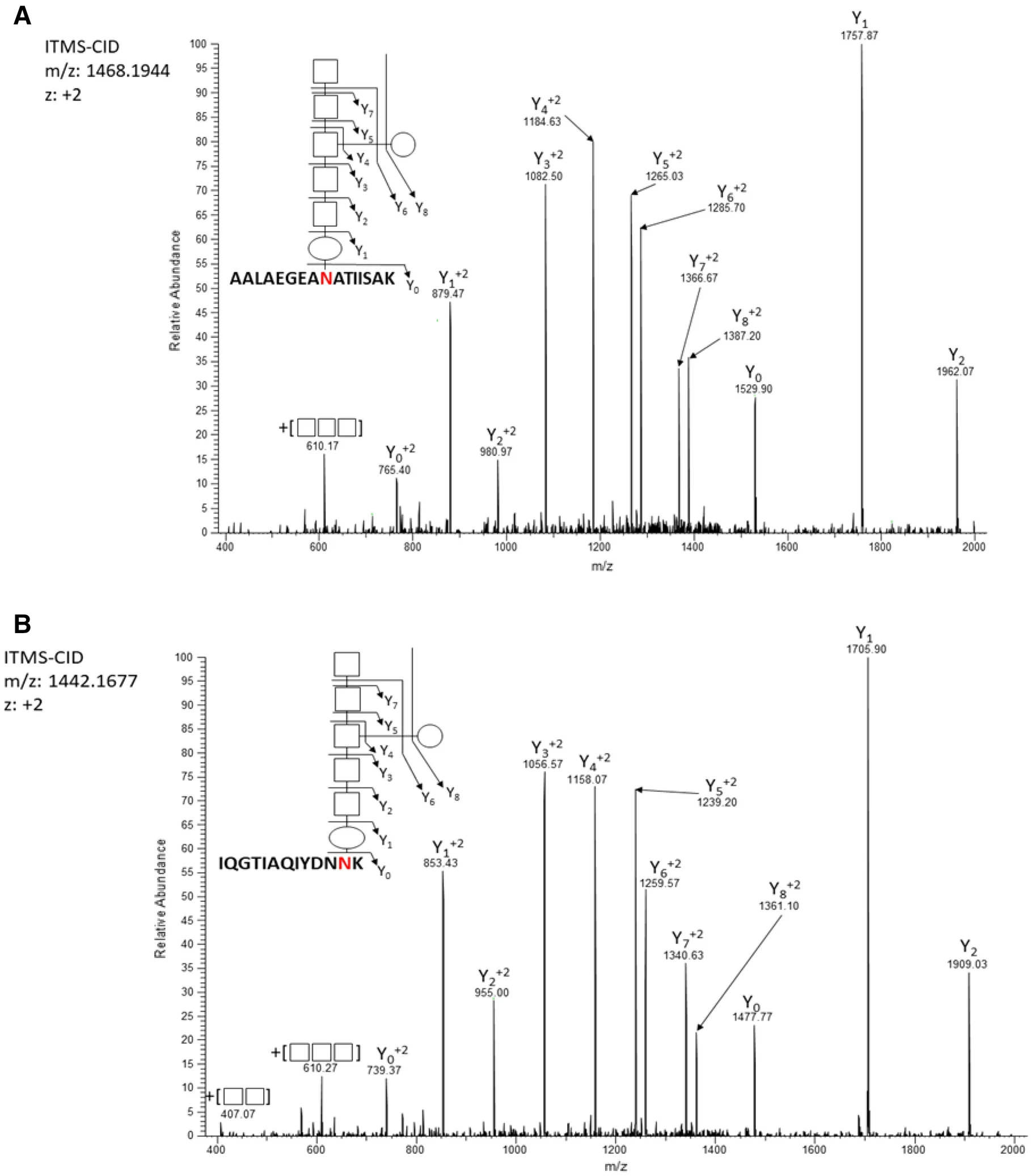
McDonald JB. et al. Sci Rep. 2023.
How to order?







