Alginic Acid Analysis Service | Pharmaceutical Excipient
Alginic acid is a natural polysaccharide derived from brown algae and is a linear polymer composed of D-mannuronic acid and L-guluronic acid residues. Due to its unique physicochemical properties, including high water absorption, gel formation, and thickening ability, alginic acid has been widely applied in the pharmaceutical industry. As a pharmaceutical excipient, it can be used as a binder and disintegrant in tablets and capsules, as a stabilizer and thickening agent in emulsions, suspensions, and gels, and it is also utilized in controlled-release systems and drug delivery research. With increasingly strict pharmaceutical quality regulations, higher requirements are being placed on the source, structure, purity, and safety of alginic acid.
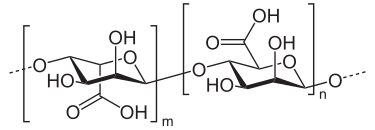
Figure 1. The Structure of Alginic Acid
As a professional pharmaceutical excipient analysis CRO, MtoZ Biolabs provides a systematic alginic acid analysis service that offers reliable testing and evaluation to support pharmaceutical companies and research institutions in drug development and regulatory compliance.
Services at MtoZ Biolabs
MtoZ Biolabs’ alginic acid analysis service covers physicochemical characterization, impurity control, quality evaluation, and functional testing, aiming to help clients comprehensively understand excipient properties and ensure stability and suitability in pharmaceutical formulations.
To achieve this, we provide a range of targeted assessments, including:
✔️ Chemical Composition and Structural Characterization: Analysis of the ratio of mannuronic acid to guluronic acid, molecular weight distribution, and degree of polymerization, with evaluation of potential degradation or structural changes.
✔️ Physicochemical Property Testing: Swelling, solubility, viscosity measurement, hygroscopicity, and crosslinking evaluation to assess performance in different formulations.
✔️ Impurity and Safety Analysis: Detection of ash, heavy metals, arsenic, lead, microbial limits, and other potential contaminants to ensure compliance with pharmacopeial and international standards.
✔️ Stability and Storage Performance: Accelerated degradation and stability tests under various temperature and humidity conditions to assess reliability during storage and use.
✔️ Functional Testing: Evaluation of performance as a binder, disintegrant, or sustained-release matrix through simulated formulation studies.
Analysis Workflow
1. Sample Receipt and Preparation: Registration and labeling, followed by drying, grinding, or dissolution if needed.
2. Physicochemical Analysis: Measurement of molecular weight, composition ratio, viscosity, and swelling properties.
3. Impurity and Safety Testing: Assessment of heavy metals, microbial limits, and ash content.
4. Functional and Stability Testing: Simulation experiments based on formulation requirements.
5. Data Integration and Report Preparation: Compilation of results into a detailed report with conclusions and recommendations.
Service Advantages
1. End-to-End Analytical Coverage
MtoZ Biolabs provides an end-to-end alginic acid analysis service that spans physicochemical characterization, impurity detection, and stability testing, giving clients a complete picture of excipient performance without missing essential quality attributes.
2. International Standard Compliance
We reference multiple pharmacopeias (USP, PhEur, BP) in our testing, ensuring results with global comparability that are suitable for R&D, regulatory submission, and quality control.
3. Advanced Platform and Multi-Technology Integration
MtoZ Biolabs combines mass spectrometry, spectroscopy, chromatography, and physicochemical testing platforms, enabling efficient and accurate analysis of the complex properties of alginic acid.
4. Customized Solutions
We flexibly tailor the testing scope and focus to client needs, whether for early-stage research, process optimization, or regulatory submission, and provide truly personalized support.
5. Efficient Execution and Report Delivery
With well-established internal project management and an experienced technical team, MtoZ Biolabs ensures timely project completion and delivers standardized reports with clear and actionable conclusions.
Applications
1️⃣ Tablet and Capsule Development: Performance evaluation as binder and disintegrant.
2️⃣ Semi-Solid and Liquid Formulations: Research on thickening and stabilizing effects in emulsions, suspensions, and gels.
3️⃣ Controlled and Sustained Release Systems: Assessment of drug release characteristics in hydrogels, microspheres, and floating dosage forms.
4️⃣ Excipient Quality Control: Ensuring alginic acid raw materials and excipients meet pharmacopeial standards.
5️⃣ Novel Applications: Exploration of alginic acid as a drug delivery carrier or tissue engineering scaffold.
FAQs
Q1: Can the analysis report be used for regulatory submission?
A1: Yes. The report content references pharmacopeial standards, and results are internationally recognized and suitable as supportive documentation for R&D and regulatory filing.
Sample Submission Suggestions
1. Samples should be pure, dry, and protected from moisture.
2. Recommended sample amount: at least 5 g (if in solution, please specify concentration and solvent).
3. For special storage conditions, please inform our technical staff in advance.
4. It is recommended to include a sample information sheet with source, batch number, and intended application.
Deliverables
1. Detailed Analytical Report: Including experimental methods, results, charts, and explanations.
2. Quality Evaluation Conclusion: Assessment of compliance against pharmacopeial or industry standards.
3. Raw Data Files: Partial raw test data available upon request.
4. Technical Recommendations: Feasibility advice for formulation development and quality improvement.
MtoZ Biolabs provides high-quality and systematic alginic acid analysis service. We look forward to working with you to ensure compliance and safety in drug development and production.
Contact our technical team for more information and customized solutions.
How to order?







