1D SDS-PAGE and IEF Services
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
SDS-PAGE Based Protein Separation Service
Submit Inquiry
How to order?


Email:
info@MtoZ-Biolabs.comEmail:
info@MtoZ-Biolabs.comMtoZ Biolabs provides comprehensive protein gel analysis services, including SDS-PAGE, IEF, and Native PAGE.
Polyacrylamide gel electrophoresis (PAGE) is a widely used technique for separating macromolecules, including DNA, RNA, and proteins. In this process, charged ions migrate under an electric field. The mobility of charged molecules is proportional to their net charge and the resistance of the solution they pass through. Sodium dodecyl sulfate (SDS), an anionic detergent, imparts a net negative charge to polypeptide chains over a broad pH range. Polypeptide chains bind to a consistent amount of SDS relative to their molecular weight, and the negative charge of the SDS disrupts most of the protein's complex structures, effectively denaturing them.
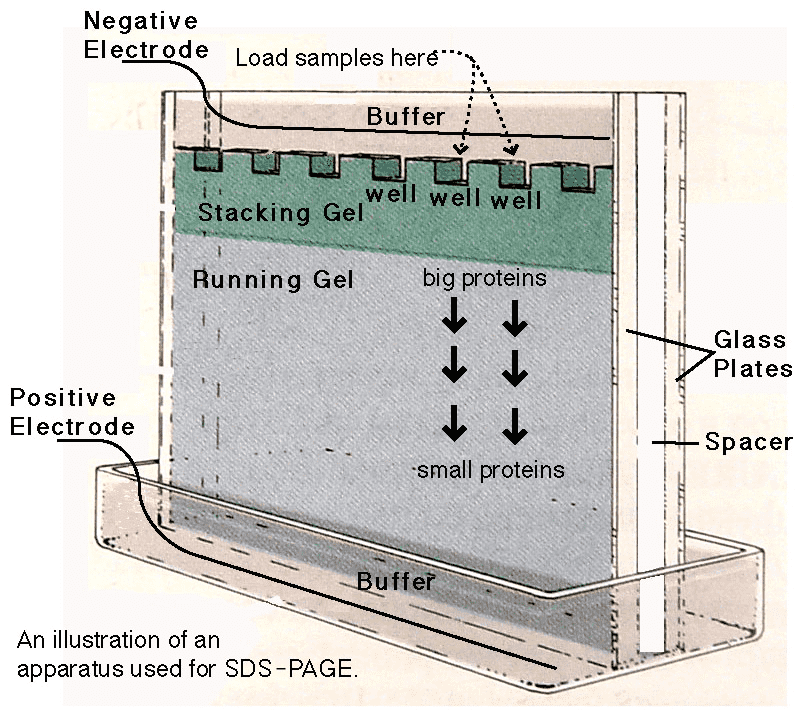
Figure 1. 1D SDS-PAGE
When a protein sample is added to the gel loading well, larger proteins may not have entered the gel yet, while smaller proteins may have already migrated beyond it. To achieve better separation, the gel is divided into two layers: a stacking gel and a separating gel. The separating gel, located at the bottom, is wider while the stacking gel above it is narrower. The electrophoresis buffer, made with glycine at pH 8.3, interfaces with the stacking gel at pH 6.8 and the separating gel at pH 8.8. During electrophoresis, negatively charged molecules migrate in the same direction. Because only about 10% of glycine ions carry a negative charge at pH 8.3, the current is mainly carried by Cl- ions in the stacking buffer, which migrate ahead of the proteins at the bottom of the stacking gel. Protein molecules coated with SDS are trapped between glycine ions above and Cl- ions below, forming a narrower band than initially loaded.
As proteins migrate into the separating gel, glycine in the separating gel buffer at pH 8.8 becomes negatively charged and migrates rapidly, closely aligning with Cl- ions, with proteins following just behind. At this stage, proteins migrate into the separating gel along the path of Cl- and glycine anions, carrying the current. Proteins become rod-like, negatively charged high-molecular-weight polymers with similar hydrodynamic properties, and their mobility depends only on molecular weight.
SDS-PAGE is widely used in proteomics, including protein molecular weight determination, protein identification, sample purity analysis, disulfide bond identification, and protein quantification.
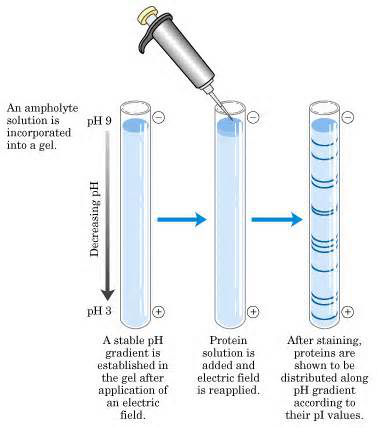
Isoelectric focusing (IEF) is an electrophoresis technique used to separate proteins based on their isoelectric points (pI). When pI equals pH, the protein's net charge is zero, so the protein does not migrate in the electric field. In IEF, separation occurs in polyacrylamide or agarose gel containing a mixture of ampholytes that migrate in the electric field and form a pH gradient in the gel. Protein molecules are concentrated in a medium with a pH gradient, generating a positively charged anode and a negatively charged cathode. Charged protein molecules move toward the opposite electrode until they reach a point where the surrounding pH matches their pI, at which point their net charge becomes zero, and they stop migrating.
Proteins with the same pI concentrate in fixed pH bands. Each protein is located at the pH gradient corresponding to its pI. IEF has extremely high resolution, allowing proteins differing by a single charge to be separated into distinct bands.
Baudin, B. Gel Electrophoresis - Principles and Basics. 2012.
Figure 2. IEF Analysis
SDS-PAGE Based Protein Separation Service
How to order?


Submit Inquiry
