Why ABPP Is Regarded as a Powerful Tool in Functional Proteomics: A Comprehensive Overview of Its Principles and Applications
Uncovering a Fundamental Challenge: Protein Expression ≠ Protein Activity
In life science research and drug development, researchers frequently encounter paradoxical observations:
1. An enzyme shows high expression levels in transcriptomic and proteomic data, yet exhibits no detectable activity during functional validation.
2. Drug treatment results in significant biological effects, even though the expression level of the target protein remains unchanged.
3. Dozens or even hundreds of candidate targets are identified through expression-based screening, but only a few are successfully validated functionally.
At the core of these discrepancies lies a crucial principle: expression does not equate to function. Conventional proteomics techniques, such as DDA, DIA, and TMT-based quantification, enable large-scale protein identification and quantification but fail to distinguish between catalytically active enzymes and their inactive, “dormant” forms.
Activity-Based Protein Profiling (ABPP) directly addresses this limitation. By employing chemical probes that selectively bind to catalytically active enzymes, ABPP adds a functional dimension to proteomic analysis. As such, it has emerged as a transformative technology in the field of functional proteomics.
Redefining Proteomics: The Functional Detection Logic of ABPP
1. The Distinctive Advantage of ABPP
ABPP is a chemical proteomics technique specifically designed to label active enzymes through covalent binding. Its underlying principle involves using structurally selective probes to covalently react with the active sites of enzymes in complex biological samples. These labeled proteins can then be identified through mass spectrometry and other detection methods.
In contrast to traditional proteomics, ABPP is distinguished by its functional focus:
(1) It does not quantify total protein levels, but instead identifies whether a protein exhibits enzymatic activity.
(2) It emphasizes catalytic function over differential expression.
(3) It selectively targets proteins that are catalytically active under physiological or pathological conditions.
2. The Probe: A Molecular Sensor of Enzymatic Activity
Each enzyme class, such as serine hydrolases, caspases, or metalloproteinases, possesses distinct structural and chemical features at their active sites. ABPP probes are rationally designed to exploit these features, enabling specific and covalent labeling of target enzymes.
A typical ABPP probe comprises three functional modules:
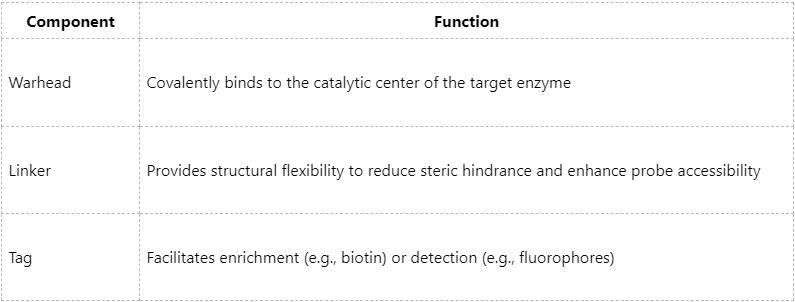
Thanks to their structural specificity, ABPP probes enable selective recognition of active enzymes, a capability that is difficult to achieve through expression-based or non-targeted proteomics approaches.
From Screening to Confirmation: Enhancing Target Discovery Efficiency with ABPP
In the context of drug development, target discovery remains one of the most challenging bottlenecks. While transcriptomic or proteomic profiling can generate hundreds of candidate targets, the success rate of functional validation is typically low.
By focusing on enzymatic activity rather than expression alone, ABPP significantly improves the “hit rate” in target discovery. Key mechanisms include:
1. Differential Activity Profiling
ABPP can be applied to compare enzyme activity between treated and control samples using identical activity-based probes. Mass spectrometry is used to quantify probe-labeled proteins:
(1) A significant decrease in probe signal for a given enzyme in the treatment group suggests competitive binding of the drug to that enzyme.
(2) An increase in enzymatic activity in a disease model may indicate the enzyme’s potential role as a pathogenic driver.
2. Competitive ABPP for Target Validation
Following initial screening, competitive ABPP experiments can be performed to validate direct drug–target interactions:
(1) The candidate drug is first incubated with the biological sample to bind potential enzyme targets.
(2) A probe is then introduced to determine whether the drug obstructs probe binding.
(3) A significant reduction in probe labeling indicates direct competition, offering strong evidence of on-target binding.
This approach is widely recognized as the gold standard for validating covalent drug–enzyme interactions.
Beyond Traditional Boundaries: Versatile Applications of ABPP
1. Target Identification and Off-Target Profiling for Small Molecule Drugs
ABPP is especially suitable for studying small-molecule interactions with enzyme targets, particularly covalent inhibitors. It enables direct confirmation of drug–target binding and early identification of potential off-target enzymes, thereby reducing the risk of toxicity.
2. Mapping Disease-Associated Enzyme Activity and Discovering Functional Biomarkers
In diseases such as cancer, autoimmune disorders, and neurodegenerative diseases, ABPP can delineate aberrant enzymatic activity across signaling pathways. This facilitates the identification of functional biomarkers associated with disease states or progression.
3. Target Discovery for Natural Products and Lead Compounds
The mechanisms of action of many natural products remain poorly understood. ABPP enables early-stage elucidation of enzyme-binding profiles, accelerating target identification and improving resource efficiency in lead compound screening.
4. Building Functional Proteome Maps and Kinetic Models
By performing ABPP under various time points or experimental conditions, researchers can reconstruct the temporal dynamics of enzymatic activity in physiological or pharmacological processes. These functional proteome maps provide a valuable foundation for systems biology modeling.
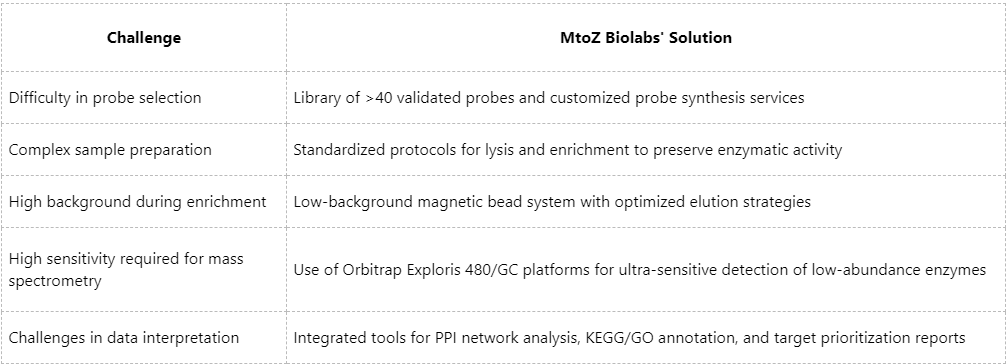
Technical Challenges and MtoZ Biolabs' End-to-End Solutions
Despite its advantages, widespread implementation of ABPP in functional proteomics still faces technical challenges. MtoZ Biolabs, a leading provider of functional proteomics services, offers comprehensive solutions spanning probe design, sample preparation, data acquisition, and interpretation.
ABPP compels us to move beyond simply asking Is a protein present?, urging deeper inquiry: Is it active? Is it functionally relevant? At this functional level, ABPP currently stands as the most mature, effective, and scalable solution. With extensive experience supporting universities, pharmaceutical companies, and clinical institutions, MtoZ Biolabs has successfully implemented ABPP-based projects across a wide range of applications, transforming functional proteomics from a theoretical concept into a robust, validated platform for both scientific discovery and clinical innovation.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







