What Is Targeted Quantitative Metabolomics?
- MRM mode: Defined precursor ion–product ion transitions are monitored to ensure high selectivity for the target metabolites.
- Internal standard quantification: Stable isotope–labeled internal standards are employed to correct for matrix effects and signal drift during analysis, thereby enhancing the accuracy and reproducibility of quantification.
- A comprehensive library of metabolite standards and internal standards
- An experienced mass spectrometry analysis team with method development expertise
- Capability to process diverse biological matrices (serum, urine, tissue, cells, etc.)
- Rigorous quality control protocols and in-depth bioinformatics reporting
Targeted quantitative metabolomics is a research strategy in metabolomics characterized by the predefined selection of metabolites and their precise quantification. This approach employs a predetermined metabolite list and leverages highly sensitive, selective analytical platforms, such as liquid chromatography–tandem mass spectrometry (LC–MS/MS) or gas chromatography–mass spectrometry (GC–MS), to achieve accurate measurements of the absolute concentrations or relative abundances of specific metabolites across diverse biological samples. Compared with untargeted metabolomics, targeted quantitative approaches offer greater specificity and reproducibility, making them particularly suitable for mechanism validation, biomarker evaluation, and pharmacodynamic studies.
Technical Principles of Targeted Quantitative Metabolomics
Targeted quantitative metabolomics typically utilizes triple quadrupole mass spectrometry (QqQ) or high-resolution mass spectrometry (e.g., Orbitrap, Q-TOF) coupled with liquid chromatography. Central to this methodology are the multiple reaction monitoring (MRM) and selective reaction monitoring (SRM) modes:
Differences Between Targeted and Untargeted Metabolomics
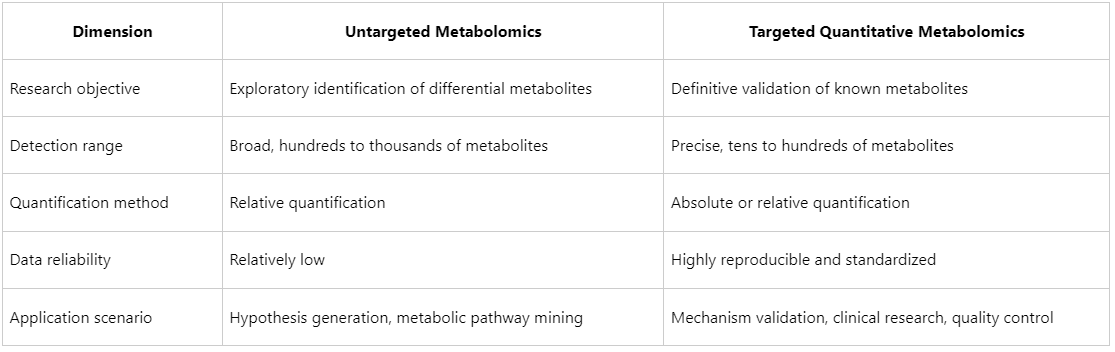
Application Scenarios of Targeted Quantitative Metabolomics
1. Biomarker Validation and Translational Research
Following the identification of candidate differential metabolites in untargeted analyses, targeted assays are employed for quantitative validation in larger cohorts, enabling the assessment of their sensitivity and specificity as disease biomarkers.
2. Pharmacodynamic and Toxicological Evaluation
Quantitative monitoring of metabolite dynamics can elucidate drug-mediated modulation of specific metabolic pathways, supporting studies on pharmacodynamic mechanisms, toxicity surveillance, and pharmacokinetics.
3. Nutrition and Metabolic Intervention Research
Targeted approaches allow precise monitoring of key nutritional metabolites (e.g., amino acids, fatty acids, vitamins) in terms of dietary intake and in vivo fluctuations, facilitating the design and evaluation of nutritional intervention strategies.
4. Compliance Testing and Quality Control
In food safety, agriculture, and industrial biotechnology, quantitative detection of target metabolites supports product quality assessment, process monitoring, and regulatory compliance verification.
Designing a High-Quality Targeted Quantitative Metabolomics Workflow
A robust targeted metabolomics study requires rigorous optimization and standardization across several stages:
1. Target Selection and Database Integration
Define the metabolite panel based on study objectives, cross-referenced with databases such as HMDB, KEGG, and LipidMaps to ensure accurate structural, retention time, and transition information.
2. Standard and Internal Standard Preparation
Selection of appropriate reference standards and internal standards, preferably stable isotope–labeled, is essential for absolute quantification and for ensuring data reliability.
3. Method Validation and Quality Control
Key analytical parameters, including linearity, limit of detection, repeatability, and recovery, should be systematically validated, with QC, blank, and replicate samples incorporated to safeguard data integrity.
4. Bioinformatics and Statistical Analysis
Multivariate statistical analyses, such as principal component analysis (PCA) and partial least squares–discriminant analysis (PLS–DA), should be employed to characterize metabolite variation patterns, alongside pathway enrichment analysis for deeper biological interpretation.
Advantages of MtoZ Biolabs in Targeted Metabolomics
MtoZ Biolabs has established a high-throughput, sensitive, and accurate mass spectrometry platform for targeted metabolomics, featuring:
With the rapid advancement of precision medicine and translational research, targeted quantitative metabolomics is emerging as a pivotal link between basic science and clinical applications. Through quantitative profiling of key metabolites, researchers can achieve higher-resolution insights into biological processes and identify novel targets for disease intervention. MtoZ Biolabs aims to leverage cutting-edge technologies to facilitate scientific advancements, ensuring that every data point yields maximal value.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







