Western Blotting Quantitative Service
Western blotting quantitative is a widely used protein detection method in molecular biology, biochemistry, immunogenetics, and biomedical research. It is commonly used to identify and analyze specific proteins in cells or tissues and provides both qualitative and semi-quantitative analysis. Coupled with chemiluminescent detection, it allows for the comparison of protein expression differences in multiple samples. The basic principle of western blotting quantitative service is to color protein samples from cell or tissue lysates that have been processed by gel electrophoresis using specific antibodies. By analyzing the color intensity and position, information about the protein expression in the analyzed cells or tissues can be obtained.
Western blotting quantitative technology is developed based on gel electrophoresis and solid-phase immunoassay techniques. Proteins are first separated by SDS-polyacrylamide gel electrophoresis, then transferred to a solid-phase support (PVDF membrane), where proteins are adsorbed non-covalently. The proteins on the solid-phase carrier act as antigens and react with corresponding primary antibodies, followed by secondary antibodies conjugated with enzymes or isotopes. Color detection with substrate reveals the specificity of proteins separated by electrophoresis. The western blotting quantitative service is characterized by high specificity and convenience, playing a crucial role in disease research, drug development, and cell signaling pathway analysis.
Services at MtoZ Biolabs
According to different samples, the western blotting quantitative service provided by MtoZ Biolabs can adopt different pyrolysis extraction schemes, extract and quantify efficiently, and combine with chemiluminescence detection to obtain the information of protein expression in the analyzed cells or tissues.
Analysis Workflow
1. Protein Extraction
Based on the sample type, appropriate protein lysis buffers and extraction methods are selected. A mixture of protease and phosphatase inhibitors is added for cell disruption and protein collection.
2. Protein Quantification
A suitable amount of undenatured protein solution is taken, and protein concentration is measured to ensure accurate protein quantification, avoiding differences in sample loading that could lead to band discrepancies and errors in results.
3. Electrophoresis
Polyacrylamide gel is used to separate proteins. The protein sample is given a negative charge in the presence of SDS, and the proteins are separated according to their molecular size in the gel by applying an electric current.
4. Transfer to Membrane
The separated proteins are transferred from the SDS-PAGE gel to a solid-phase carrier, typically a PVDF membrane. This transfer is usually done by either electrotransfer (passing current through the gel and membrane) or wet transfer (using capillary action). The transferred gel is then stained with Coomassie Brilliant Blue to check for successful and complete transfer.
5. Blocking
Non-specific protein binding sites on the membrane are blocked with non-specific proteins (skim milk or BSA) to prevent non-specific antibody binding and improve specificity.
6. Antibody Incubation
The membrane is incubated with a specific primary antibody that binds to the target protein. Unbound primary antibody is removed by washing, followed by incubation with the corresponding secondary antibody, which is tagged with either fluorescence or enzyme labels.
7. Detection
The label on the secondary antibody allows the target protein to be visualized after substrate addition. The substrate reacts with the enzyme to produce luminescence or color change. The signal is captured by appropriate imaging devices to visualize the detected protein bands. The position of the band represents the protein's size, and the intensity of the band reflects the relative quantity of the protein.
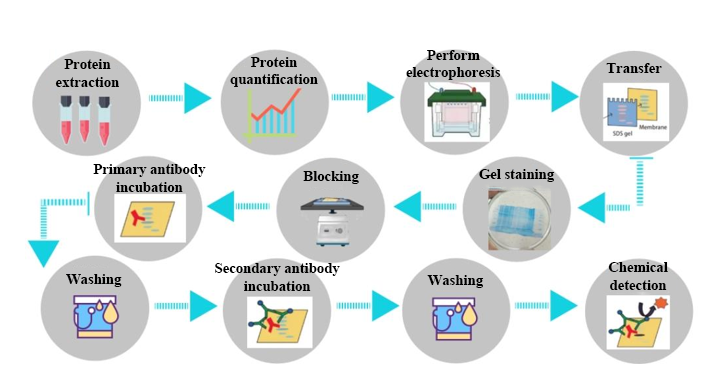
Figure 1. Western Blotting Quantitative Service Process.
Precautions
1. Before transferring proteins to the membrane, ensure to activate the membrane with methanol to ensure the accuracy and stability of the experiment.
2. During the membrane, gel, and filter paper sandwiching process, carefully remove any air bubbles. Air bubbles may cause incomplete transfer of proteins to the membrane, affecting the accuracy of the experiment.
3. Avoid storing protein samples for long periods, as this may lead to protein degradation, affecting the accuracy of experimental results. Samples should be processed promptly to ensure protein stability and experimental validity.
FAQ
Q1. Background is dirty.
A: Reduce the sample loading volume, lower the primary antibody concentration, adjust the incubation time and temperature of the primary antibody, and increase the blocking solution concentration or incubation time.
Q2. Weak target band.
A: Increase the sample loading volume and adjust the dilution ratio of the primary antibody.
Q3. Multiple bands, target band not specific.
A: The primary antibody may be binding non-specifically to other proteins, so try replacing the primary antibody. It’s also possible that the primary antibody concentration is too high.
Q4. White ring at the edges of bands.
A: There were air bubbles between the membrane and gel during the transfer process.
Q5. Trailing bands.
A: The protein amount is too high, the primary antibody concentration is too high or the incubation time is too long, or there may be some protein degradation in the sample.
Service Advantages
1. Advanced Analytical Platform
MtoZ Biolabs has established an advanced western blotting quantitative service platform, ensuring reliable, fast, and high-precision analysis.
2. Transparent Pricing
Our pricing is transparent, with no hidden or additional fees.
3. Customized Research Solutions
MtoZ Biolabs offers customized services to solve your unique research issues and experimental requirements.
Sample Submission Suggestions
1. Sample Types
We accept microbial, animal, and plant tissue samples, as well as cell samples. We ensure that the samples are properly preserved to maintain protein integrity and spatial distribution.
2. Sample Amount
We recommend preparing more than 3 biological replicates.
*Note: If you have any special requirements or need assistance with sample preparation, please contact us.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Results Report
4. Raw Data Files
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Our ultimate aim is to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption. Free project evaluation, welcome to learn more details!
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Quantitative Proteomics Service
How to order?







