Vaccine Design and Development Service | Cryo-EM
The development of effective vaccines relies on a thorough understanding of antigen structure, epitope accessibility, and the physicochemical stability of delivery systems. As vaccine technologies evolve—from traditional inactivated formulations to new vaccine forms such as subunit vaccines, virus-like particles (VLPs), mRNA-loaded lipid nanoparticles (LNPs), and viral vectors—the structural complexity of these modalities demands advanced analytical approaches. High-resolution structural characterization is critical not only for elucidating the molecular basis of immunogenicity but also for guiding rational antigen design, optimizing formulation parameters, and ensuring batch-to-batch consistency throughout the development pipeline.
Cryogenic electron microscopy (Cryo-EM) has become an indispensable tool in vaccine design and development, offering near-native imaging of macromolecular complexes, nanoparticle carriers, and multimeric antigen structures. Its ability to preserve biological specimens in a vitrified state enables direct visualization of conformation-sensitive epitopes, antigen-nanocarrier interfaces, and formulation stability under various storage or stress conditions.
Service at MtoZ Biolabs
MtoZ Biolabs offers a dedicated Vaccine Design and Development Service powered by Cryo-EM. Our Vaccine Design and Development Service delivers critical structural insights to accelerate antigen engineering, support formulation optimization, and strengthen regulatory submissions across preclinical and clinical development stages. Cryo-EM works by vitrifying biological samples in a thin layer of amorphous ice, preserving their native architecture. Applied to vaccine design and development, Cryo-EM enables:
💠Antigen Structural Characterization: Direct visualization of antigen conformation, epitope exposure, and multimerization.
💠Nanoparticle Formulation Analysis: Morphological assessment of VLPs, mRNA-LNPs, and viral vectors.
💠Stability and Integrity Monitoring: Detection of aggregation, degradation, or structural changes during storage or under stress.
💠Comparability Studies: Structural verification before and after formulation adjustments or manufacturing changes.
💠Quality Control Support: High-resolution confirmation of critical structural attributes aligned with regulatory requirements.
These capabilities provide critical data for guiding antigen engineering, formulation refinement, stability optimization, and regulatory documentation.
Analysis Workflow
1. Consultation and Study Design
We work with clients to define project goals, target structural attributes, and Cryo-EM analysis strategies tailored to vaccine type and development stage.
2. Sample Preparation and Vitrification
Samples are vitrified under optimized conditions to preserve native morphology and minimize artifacts.
3. Cryo-EM Imaging and Data Collection
High-resolution images are acquired under low-dose conditions using state-of-the-art direct electron detectors.
4. Structural Analysis and Interpretation
Comprehensive analysis includes antigen conformation assessment, nanoparticle morphology evaluation, aggregation profiling, and stability monitoring.
5. Report Delivery
Clients receive detailed reports containing representative images, quantitative metrics, and expert interpretation to support vaccine design and development decisions.
Service Advantages
✅Supports Multiple Vaccine Types: Our Cryo-EM service is applicable to both traditional vaccines (e.g., inactivated or attenuated) and new vaccine forms such as subunit vaccines, VLPs, viral vectors, and mRNA–LNP systems.
✅Expert Scientific Support: Each project is guided by a multidisciplinary team with expertise in vaccine research, structural biology, and nanoparticle analysis.
✅Flexible and Efficient Execution: We tailor every project to match the client’s vaccine modality, development stage, and data needs—ensuring efficiency without compromising analytical depth.
✅One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Applications
1. High-Resolution Structural Analysis and Antigen Design
Our Cryo-EM service enables near-atomic resolution imaging of vaccine antigens, facilitating the identification of epitope conformation, oligomerization states, and surface topology. These insights support rational antigen design and the engineering of stabilized immunogens for enhanced efficacy.
2. Immunogenicity Research
We provide structural data that help correlate antigen integrity with immunogenic potential. By visualizing how antigens are presented on delivery platforms, Cryo-EM supports the development of vaccines with optimized immune recognition and response profiles.
3. Vaccine Carrier Characterization
We provide structural evaluation of diverse vaccine delivery systems, including lipid nanoparticles (LNPs), virus-like particles (VLPs), and recombinant viral vectors. Cryo-EM enables direct visualization of particle morphology, size distribution, surface architecture, and cargo encapsulation, supporting carrier optimization and quality control across development stages.
4. Personalized Vaccine Formulation Support
For individualized or precision vaccine strategies, we support structural evaluation of custom-designed antigens, patient-specific epitope displays, or modified nanoparticle formulations, enabling structure-based refinement of personalized immunotherapies.
Case Study
Cryo-EM Characterization of a Stabilized SARS-CoV-2 Spike Trimer for Vaccine Development
The SARS-CoV-2 spike (S) protein is the principal target of neutralizing antibodies and a key antigen in COVID-19 vaccine design and development. However, its intrinsic metastability—common to class I fusion proteins—leads to premature conformational shifts from the immunogenic prefusion to the non-immunogenic post-fusion state. To overcome this challenge, researchers applied structure-guided design to develop a soluble S trimer variant (S-closed) with improved conformational stability and expression yield. Using a minimal set of interprotomeric mutations, including point substitutions and disulfide bridges, the team generated an S-closed construct with 6.4-fold higher expression than its parental form and no need for a heterologous trimerization tag. High-resolution cryo-EM imaging confirmed that the engineered trimer maintained a predominantly closed prefusion conformation, preserving key neutralizing epitopes. This study demonstrates the essential role of cryo-EM in verifying antigen structure during rational vaccine design, supporting the development of more stable and immunogenic SARS-CoV-2 spike-based vaccines.
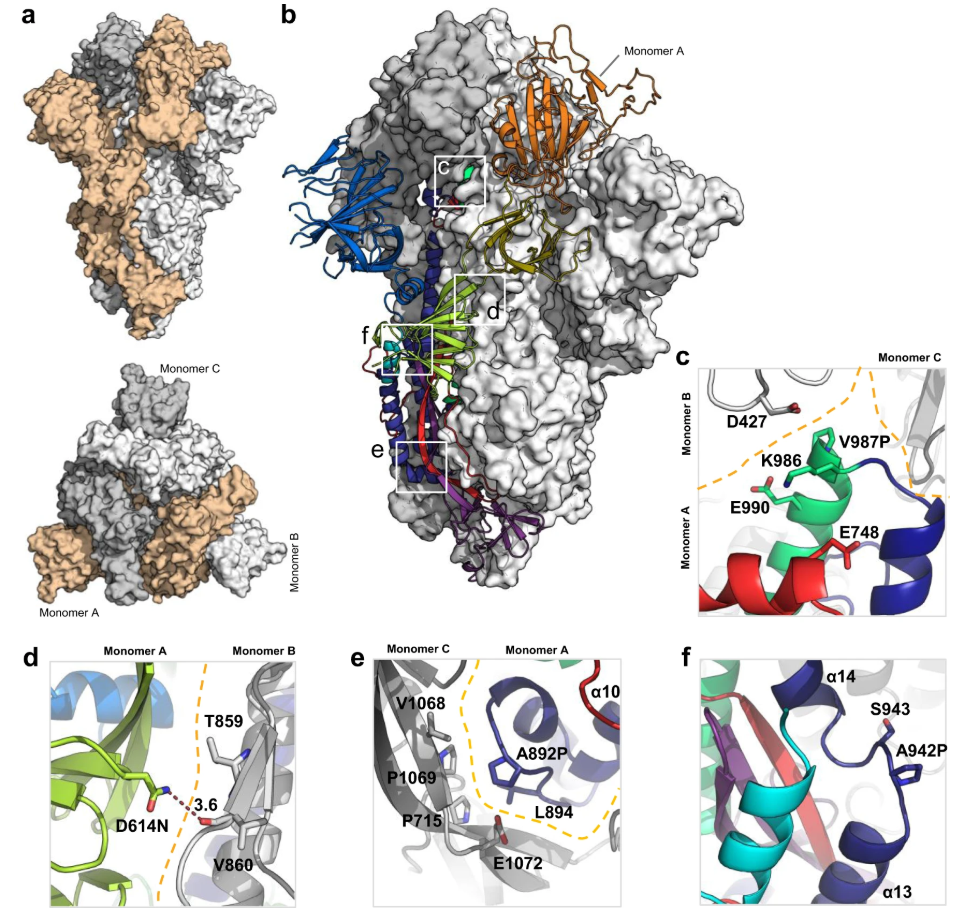
Figure 1. Structural Characterization of S-closed-Fd
FAQ
Q: Can Cryo-EM distinguish between empty and filled viral vectors or LNPs?
In many cases, yes. Cryo-EM can detect differences in internal density, helping to estimate encapsulation status, especially when combined with particle statistics.
MtoZ Biolabs empowers vaccine developers with Cryo-EM-based structural solutions to accelerate the creation of safe, effective, and stable vaccines. To learn more about our Vaccine Design and Development Service utilizing Cryo-EM, or to discuss your specific vaccine research needs, please contact us.
How to order?







