Using ABPP for Small Molecule Screening and Mechanistic Studies
In the early stages of drug discovery and mechanism-of-action (MoA) research, traditional high-throughput screening (HTS) approaches can identify active compounds from large chemical libraries. However, they often fail to address a critical question: How does the compound exert its biological effect? This is precisely where Activity-Based Protein Profiling (ABPP), a proteomics technique based on active-site-directed chemical labeling, offers unique advantages.
ABPP is a chemical probe-based strategy extensively applied in small molecule screening, target identification, and MoA elucidation. Its central principle involves the use of active-site-directed probes to selectively label functionally active enzymes or protein families, followed by proteomic analysis. Unlike conventional methods that depend on protein abundance, ABPP directly captures proteins in their active state, making it a powerful tool for functional screening, target deconvolution, and mechanistic investigation.
With the emergence of covalent inhibitors and targeted protein degradation technologies, increasing emphasis is placed on the controllability of a compound’s mode of action. As a bridge linking protein functional states to small molecule engagement, ABPP is rapidly gaining traction. It holds promise for generating high-throughput, high-resolution functional proteomic maps. Looking ahead, leveraging ABPP in conjunction with chemical genetics, AI-assisted structure prediction, and single-cell mass spectrometry represents a frontier in redefining early-stage drug discovery.
Three Key Components of an Activity-Based Probe (ABP)
1. Reactive Group (Warhead)
Covalently binds to the active site of target enzymes; commonly used groups include fluorophosphonates, epoxides, and sulfonyl fluorides.
2. Linker
Modulates the spatial conformation of the probe to reduce non-specific interactions.
3. Tag
Enables detection (e.g., fluorophores, photo-reactive groups) or enrichment (e.g., biotin) in downstream analysis.
Applications of ABPP in Small Molecule Screening
1. Selection of an Appropriate ABPP Probe
ABPP probes typically consist of three functional modules:
(1) Reactive Group (Warhead): Specifically targets the active site (e.g., fluorophosphonate for serine hydrolases).
(2) Linker: Provides spatial flexibility to facilitate effective binding.
(3) Tag: Used for visualization (e.g., fluorophores, biotin) or for click chemistry-based conjugation (e.g., alkyne/azide groups).
2. Design of Competitive ABPP Assays
Incubate the test small molecule with cell lysates or purified enzymes, followed by addition of the ABPP probe. If the compound binds to the active site of the enzyme, it will competitively inhibit the probe from labeling the site, resulting in a decreased signal.
3. Detection and Screening
Assess probe binding through SDS-PAGE combined with fluorescence imaging, biotin labeling followed by Western blotting, or quantitative mass spectrometry. By comparing signal differences in the presence and absence of the compound, one can determine its inhibitory potential.
Simplified Workflow
1. Apply the probe to biological samples (cell/tissue lysates or in situ).
2. Allow covalent binding to target proteins.
3. Enrich labeled proteins and identify them via mass spectrometry.
Strategic Applications of ABPP in Small Molecule Research
1. Competitive ABPP: Identifying Compound Targets
In this approach, the compound is incubated with the system prior to probe addition. If the compound occupies the enzyme’s active site, the probe’s labeling is diminished. Quantitative proteomic comparison across treatment conditions enables:
(1) Identification of enzyme targets that directly compete with the compound.
(2) Elucidation of the compound’s mechanism of action and selectivity.
2. Reactivity Profiling: Mapping Functional Enzymes
ABPP enables the generation of activity-based proteomic maps across tissues, cell types, or pathological states. These profiles provide functional context for compounds with unknown targets and aid in hypothesizing potential modes of action.
3. Optimization of Enzyme Family Selectivity
In covalent inhibitor development, ABPP is especially useful for identifying molecules with selective inhibition across enzyme family members.
Technical Advantages of ABPP Coupled with Mass Spectrometry
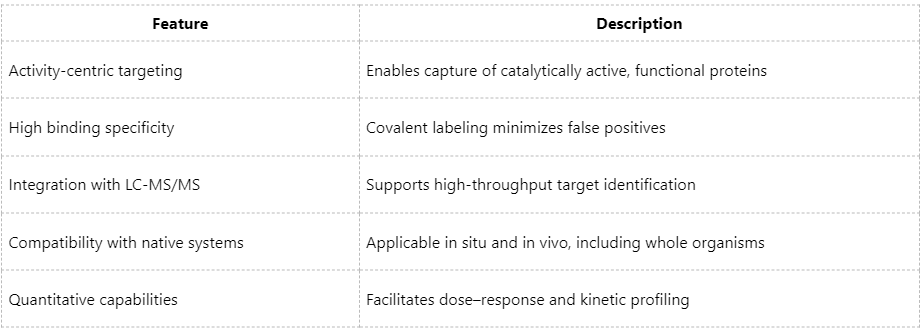
Key Considerations
1. Probe selection should be tailored to enzyme class (e.g., serine hydrolases, cysteine proteases, oxidoreductases).
2. The cell permeability and metabolic stability of the small molecule significantly influence assay outcomes.
3. Non-specific binding and background signals must be rigorously controlled.
At MtoZ Biolabs, we leverage high-resolution Orbitrap-based platforms to offer advanced quantitative ABPP solutions, including ABPP combined with TMT multiplex labeling and SILAC-based stable isotope labeling. Our integrated platforms, spanning ABPP, DIA quantification, and phosphoproteomics, enable precise interrogation of small molecule–enzyme interactions. We are dedicated to accelerating hypothesis-driven research, facilitating target discovery, and empowering innovation in life sciences and drug development.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







