Therapeutic Applications Service of Exosomes
- Tumor-targeting exosomes for siRNA or chemotherapeutic delivery (e.g., doxorubicin, paclitaxel)
- Surface-engineered exosomes for recognition of EGFR- or HER2-positive tumors
- Modulation of tumor microenvironment and inhibition of immune evasion
- MSC exosomes promote osteogenesis, cardiac tissue repair, and corneal wound healing
- Regulation of signaling pathways involved in angiogenesis and collagen remodeling
- Serve as safer alternatives to stem cell transplantation
- Delivery of neurotrophic factors (e.g., BDNF) across the blood-brain barrier
- Anti-Tau/α-synuclein therapies for Alzheimer’s and Parkinson’s disease
- Attenuation of neuroinflammation following stroke or encephalitis
- Induction of regulatory T cells and suppression of hyperactive T cells
- Anti-inflammatory effects in models of rheumatoid arthritis and inflammatory bowel disease
- Modulation of cytokine profiles to restore immune homeostasis
- Exosome-based platforms for mRNA vaccine delivery
- siRNA delivery to suppress viral replication (e.g., HIV, SARS-CoV-2)
- Design of exosome-derived nanovaccines with enhanced antigen stability and immunogenicity
Exosomes are nanoscale extracellular vesicles (EVs), typically 30–150 nm in diameter, enclosed by a lipid bilayer and secreted by various cell types. They are ubiquitously present in biological fluids and encapsulate a broad spectrum of bioactive molecules, including miRNAs, mRNAs, proteins, and lipids. Through mechanisms such as endocytosis and membrane fusion, exosomes serve as intercellular communication vehicles. Growing insights into their biological properties have highlighted their therapeutic potential in disease diagnosis, tissue regeneration, immune modulation, and drug delivery. As such, exosome-based therapeutics have emerged as a rapidly expanding area in biomedical research.
Owing to their intrinsic biocompatibility, low immunogenicity, excellent membrane permeability, and natural tissue-targeting capabilities, exosomes represent a promising platform for drug delivery and extracellular therapeutic interventions. Significant therapeutic outcomes have been reported in various disease models, including cancer, neurodegenerative disorders, autoimmune conditions, cardiovascular diseases, and applications in tissue engineering. Systematic exploration of exosome-based therapies and their translational potential in clinical applications holds both theoretical value and translational relevance.
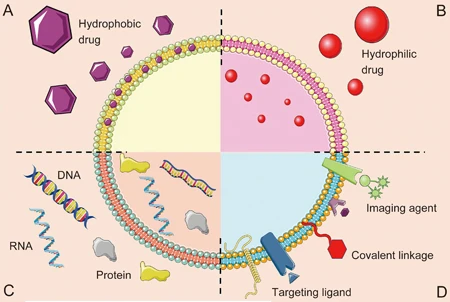
Luan, X. et al. Acta Pharmacol Sin. 2017.
Figure1. Schematic Representation of the Different Types of Exosomes Drug Delivery Systems
Service at MtoZ Biolabs
As a globally recognized service provider in exosome research, MtoZ Biolabs offers a comprehensive and systematic suite of services for therapeutic exosome development. Powered by our advanced multi-omics platforms, nanoscale biocharacterization tools, and established engineering and functional validation workflows, our services span key stages from exosome source screening and isolation to functional enhancement, delivery strategy optimization, and in vitro/in vivo efficacy evaluation. Technologies integrated into our platform include LC-MS/MS, next-generation sequencing (NGS), ultracentrifugation, nanoparticle tracking analysis (NTA), flow cytometry, and animal model validation—enabling customized, end-to-end solutions for exosome-based therapy research.
Technical Principles
Exosomes function as naturally derived nanocarriers with unique biological features that endow them with substantial therapeutic potential. Mechanistically, exosomes operate through two primary modes of action: (1) as carriers for therapeutic agents, including miRNAs, siRNAs, proteins, small molecules, or drugs, enabling targeted delivery via active or passive mechanisms; and (2) by exerting intrinsic therapeutic effects through parent cell-specific bioactive molecules—for instance, stem cell-derived exosomes are enriched with regenerative or anti-inflammatory factors that promote tissue repair and immunomodulation. Through bioengineering, exosomes can be further optimized by surface protein modification, cargo enrichment, and controlled delivery strategies, thereby enhancing their targeting precision and therapeutic efficacy.
Analysis Workflow
MtoZ Biolabs provides a fully integrated workflow for exosome-based therapeutic research, from cell source selection to efficacy assessment. The following outlines the standard process, which can be flexibly adapted to meet specific project goals:
1. Selection of Cell Source and Exosome Harvesting
Suitable donor cells—including mesenchymal stem cells (MSCs), dendritic cells (DCs), tumor cells, or peripheral blood mononuclear cells (PBMCs)—are selected according to the research objective. Culture conditions are optimized to maximize exosome yield. Both customer-provided cell lines and pre-isolated exosomes are supported.
2. Exosome Isolation and Purification
High-purity exosomes are isolated using ultracentrifugation, density gradient separation, membrane filtration, or size exclusion chromatography (SEC). Characterization includes nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and Western blot or ELISA-based surface marker validation.
3. Cargo Loading and Exosome Engineering (Optional)
Multiple loading strategies—including electroporation, sonication, co-incubation, and chemical conjugation—are available for efficient encapsulation of nucleic acids, proteins, and small-molecule drugs. Targeting can be enhanced via membrane surface engineering (e.g., RVG, RGD, HER2 peptides).
4. In Vitro Functional Validation
Bioactivity is assessed through a series of assays, including cellular uptake, cytotoxicity, signal transduction pathway analysis, inflammatory cytokine profiling, and inhibition of migration or invasion. Fluorescent labeling and luciferase reporter systems are available for mechanistic tracking.
5. In Vivo Therapeutic Evaluation (Optional)
Exosomes are administered in animal models such as mice, rats, or zebrafish, employing routes like intravenous, intranasal, or local injection. Disease models include tumor xenografts, myocardial infarction, stroke, and chronic inflammation. Pharmacodynamics, biodistribution, targeting efficiency, and safety are comprehensively evaluated.
6. Multi-Omics Mechanistic Analysis (Optional)
Proteomics (LC-MS/MS), transcriptomics (NGS), and metabolomics are employed to dissect the mechanistic basis of exosome function, offering rich datasets for therapeutic pathway elucidation and target discovery.
Applications
Exosome-based therapeutics represent a versatile and interdisciplinary strategy applicable across numerous disease models. Key applications include:
1. Cancer Therapy and Drug Delivery
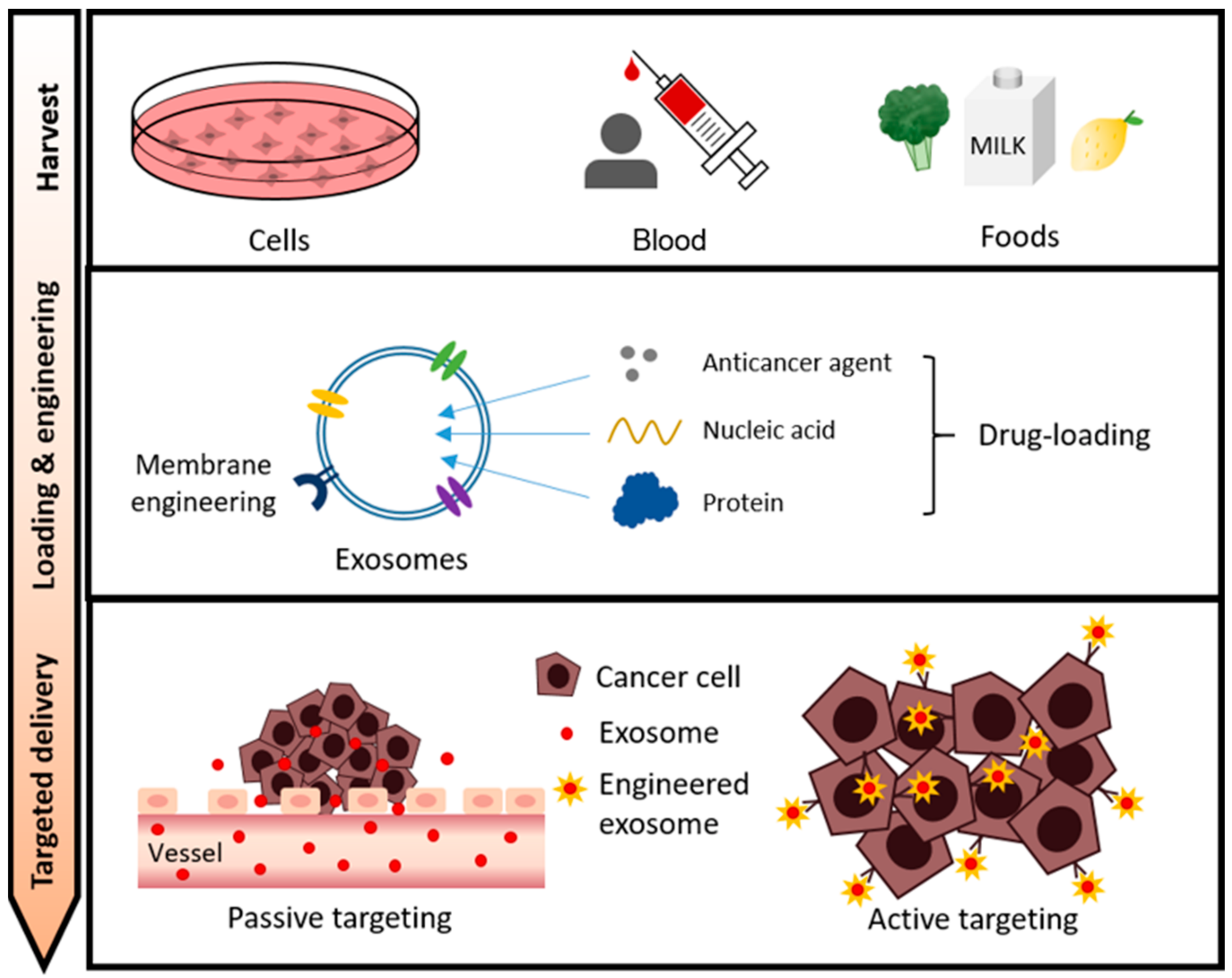
Kim, H. et al. Cancers. 2021.
Figure2. The Overall Process of Exosome-based Drug Delivery in Cancer Therapy
2. Tissue Regeneration via Stem Cell-Derived Exosomes
3. Neurodegenerative and Neurological Disease Treatment
4. Autoimmune and Inflammatory Disorders
5. Antiviral Strategies and Vaccine Development
Service Advantages
MtoZ Biolabs offers a state-of-the-art exosome therapeutic research platform with the following strengths:
✅ Rigorous Purification and Characterization Protocols
Integration of SEC, ultracentrifugation, density gradients, NTA, TEM, and Zeta potential analysis ensures exosome integrity, surface protein preservation, and functionality.
✅ Versatile Cargo Loading and Engineering Capabilities
Accommodates nucleic acids, small molecules, and proteins. Customizable engineering strategies enhance targeting ability, membrane permeability, and in vivo stability.
✅ Tailored Project Design with Full Data Support
Study plans customized to disease context and research objectives. Comprehensive data packages include bioinformatic analysis, omics interpretation, and functional validation.
Frequently Asked Questions (FAQs)
Q1: Can exosomes be extracted from user-provided cells?
A1: Yes. We support client-supplied cell lines or exosome materials and offer downstream services including purification, engineering, and functional evaluation.
Q2: Is it possible to load specific proteins or small molecules into exosomes?
A2: Yes. We offer electroporation, co-incubation, and chemical conjugation methods, with subsequent validation of loading efficiency and bioactivity.
Q3: Are exosome therapies applicable in preclinical animal studies?
A3: Absolutely. We provide platforms including mice, zebrafish, and immunodeficient models for pharmacodynamic evaluation, biodistribution analysis, and toxicology studies.
How to order?







