Targeted Drug Delivery Systems Service
Targeted drug delivery systems (TDDS) are an advanced strategy for precisely delivering drugs to specific tissues or cells, aiming to enhance therapeutic efficacy while minimizing systemic toxicity. By integrating nanotechnology, pharmaceutical chemistry, and bioengineering, TDDS effectively overcomes challenges associated with traditional drug delivery, such as uneven distribution, strong off-target effects, and short half-life. This system is widely applied in cancer therapy, neurological diseases, infectious diseases, and personalized medicine.
TDDS primarily consists of the following core components:
1. Drug carriers (such as liposomes, polymeric nanoparticles, inorganic nanomaterials, and exosomes) that encapsulate and transport therapeutic agents.
2. Targeting mechanisms (including passive targeting, active targeting, and stimulus-responsive targeting) to ensure precise drug delivery to target tissues.
3. Controlled release systems (such as pH-responsive, enzymatic-triggered, or light/magnetic field-controlled release) to optimize drug efficacy and sustained release.
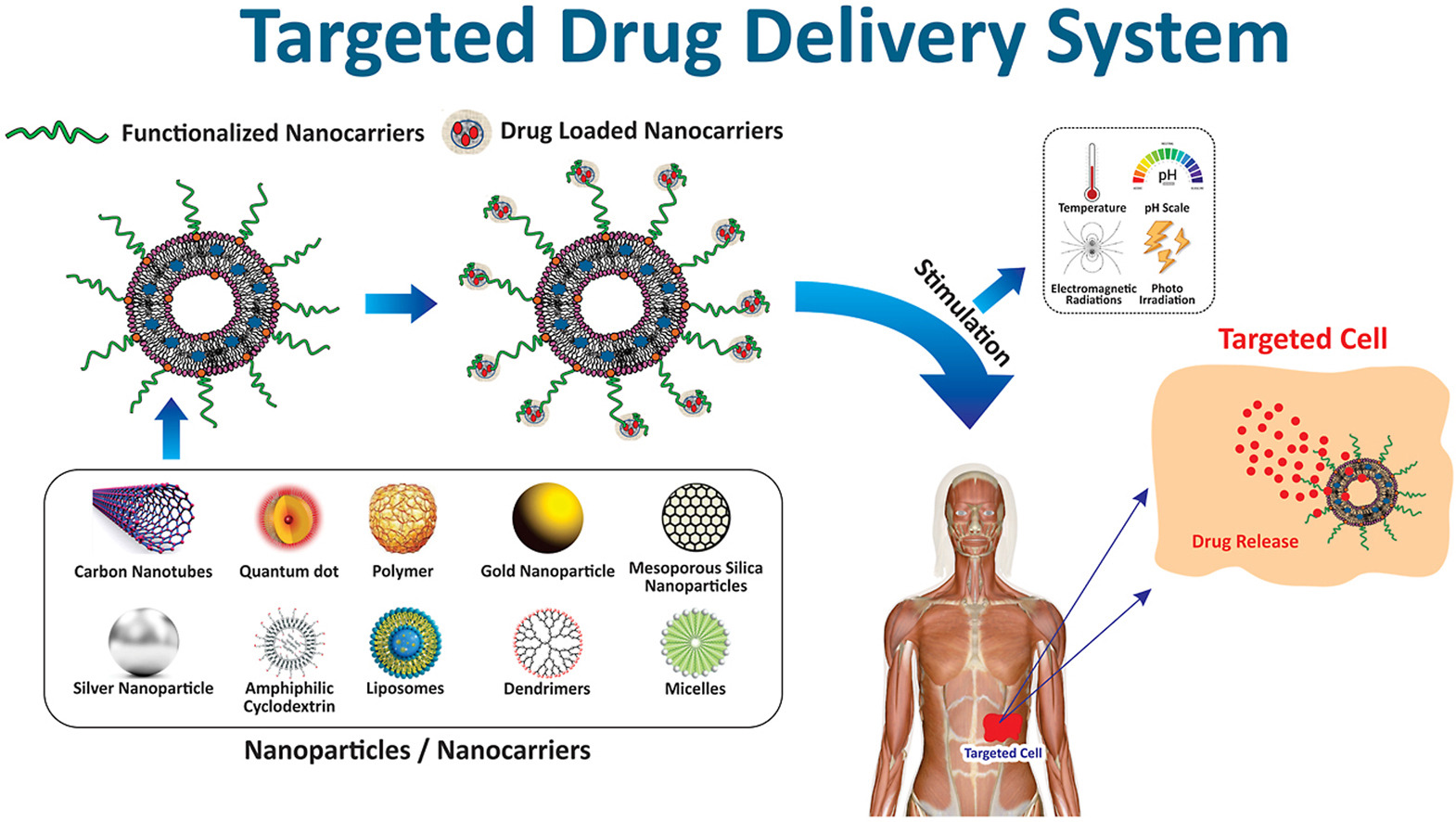
Afzal, Shah. et al. J Drug Deliv Sci Technol. 2021.
Figure 1. The Composition of Targeted Drug Delivery Systems
MtoZ Biolabs, with its advanced nanodelivery platform and mature bioengineering technologies, provides efficient and precise targeted drug delivery systems service for pharmaceutical companies and research institutions, supporting the development of innovative therapies and their clinical translation. If you are interested in our service, please contact us freely.
Service Advantages
1. Advanced Nanotechnology for Enhanced Drug Delivery Efficiency
MtoZ Biolabs utilizes cutting-edge nanomaterials and miniaturized devices to develop targeted drug delivery systems, improving drug stability and targeting efficiency in vivo. Compared to conventional drug delivery methods, the system developed by MtoZ Biolabs reduces off-target accumulation, minimizes side effects, and extends drug circulation half-life, ultimately enhancing therapeutic outcomes.
2. Precision Targeting for Increased Therapeutic Specificity
Incorporating the latest advancements in molecular pharmacology, our targeted drug delivery systems service enables efficient and precise drug transport to key cells or tissues involved in disease progression. By employing active and passive targeting strategies, including ligand modifications and stimuli-responsive release mechanisms, our system ensures accurate drug delivery to the target site, improving therapeutic specificity while reducing systemic exposure.
3. Multifunctional Integrated Platform for Personalized Drug Development
MtoZ Biolabs offers a comprehensive targeted drug delivery systems service platform that integrates smart delivery systems, biocompatibility optimization, and biodegradable carrier design to support various drug delivery needs. By leveraging high-throughput screening and bioengineering technologies, our system provides automation, efficiency, and precise control, facilitating personalized drug development and advancing next-generation precision therapies.
Applications
1. Targeted Drug Delivery Systems for Precision Medicines
Manzari, MT. et al. Nat Rev Mater. 2021.
Figure 2. Targeted Drug Delivery Systems for Precision Medicines
2. Targeted Drug Delivery Systems for Cancer Therapy
Sun, X. et al. Cancers. 2022.
Figure 3. Targeted Drug Delivery Systems in Breast Cancer
3. Targeted Drug Delivery Systems for Diabetes
Chen, X. et al. Drug Deliv. 2023.
Figure 4. Targeted Drug Delivery Systems for Diabetic Kidney Disease (DKD)
FAQ
1. What are the main barriers to the clinical translation of targeted drug delivery systems?
Despite the significant potential of targeted drug delivery systems in cancer therapy, their clinical translation faces multiple challenges. First, biological barriers such as hepatic clearance, renal excretion, and high interstitial pressure in the tumor microenvironment limit the efficiency of nanoparticle accumulation at tumor sites. Second, the heterogeneous behavior of nanocarriers in vivo, including the formation of a protein corona, affects targeting efficacy and drug release. Additionally, the effectiveness of active targeting strategies (e.g., ligand modifications) is significantly lower in vivo than in vitro, partly due to dynamic target expression and non-specific interactions. Lastly, large-scale manufacturing and quality control remain technical bottlenecks, as the lack of standardized production processes leads to batch-to-batch variability, further hindering clinical application.
2. How can nanocarrier design be optimized to improve clinical success rates?
To enhance the clinical translation of targeted drug delivery systems, several design optimizations are necessary. First, improving biological stability through PEGylation, smart-responsive materials, or surface engineering can reduce immune clearance and extend circulation time. Second, optimizing targeting strategies, such as dual or multi-targeting approaches (e.g., tumor microenvironment pH responsiveness combined with receptor-mediated targeting), can enhance tumor accumulation. Additionally, refining drug loading and release mechanisms by incorporating controlled-release technologies (e.g., pH-sensitive or enzyme-triggered drug release) ensures precise drug activation at tumor sites. Finally, advancing standardization in production and preclinical evaluation by establishing compliant large-scale synthesis processes and employing high-throughput screening to optimize formulations can enhance reproducibility and accelerate clinical adoption.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Relevant Liquid Chromatography and Mass Spectrometry Parameters
4. The Detailed Information of Results
5. Mass Spectrometry Image
6. Raw Data
How to order?







