Target Identification and Validation Service | CRO Service
- In-depth literature mining
- Gene expression profiling
- Cell-based functional assays
- In vivo disease models to examine physiological relevance
- Computer-aided target identification
- Medicinal chemistry research to assess chemical tractability and druggability
- CRISPR-Cas9 knockout/knock-in
- siRNA, or shRNA
- Silence or activate the target gene in cell-based systems or animal models
- Phenotypic screen
- Target deconvolution
- We accept a wide range of biological materials, including Cultured cells, tissues, body fluids, RNA or protein extracts, and other biological samples.
- Samples should be stored at -80℃ and shipped with dry ice to ensure the stability
The identification and validation of therapeutic targets form the cornerstone of modern drug discovery. A therapeutic target refers to a specific molecular entity—typically a protein, gene, RNA, or signaling pathway—that plays a critical role in disease pathogenesis. Modulating this target through a drug candidate is expected to induce a therapeutic effect with minimal adverse consequences. MtoZ Biolabs offers a comprehensive Target Identification and Validation Service tailored to meet the evolving needs of preclinical drug discovery. The Target Identification and Validation Service combines cutting-edge omics technologies, high-throughput screening systems, and expert bioinformatics analysis, empowering researchers to move from mechanistic hypothesis to well-validated targets. Our service is designed to not only generate high-resolution biological data but also provide strategic insight into target relevance, druggability, and downstream development potential.
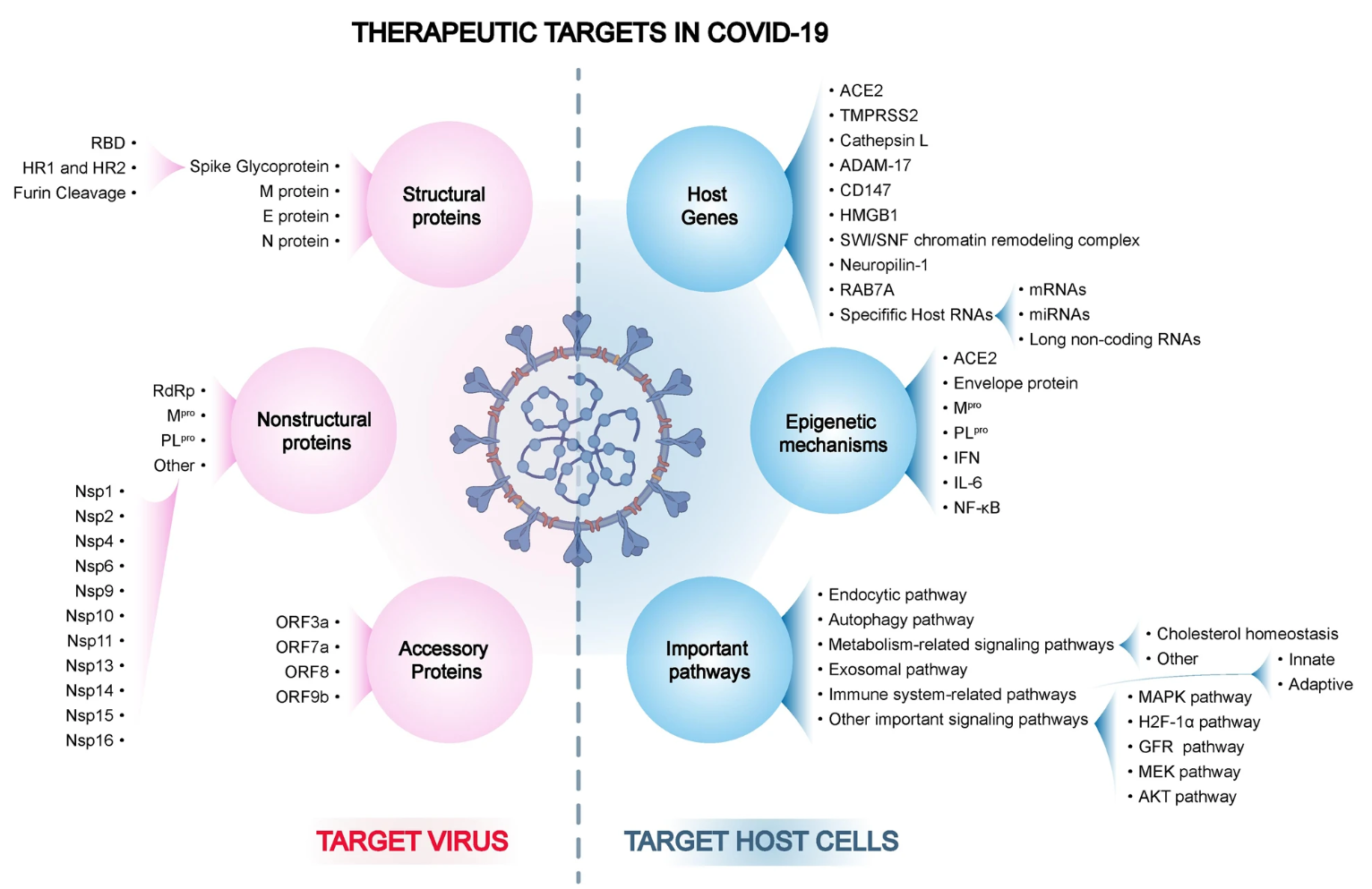
Figure 1. The Overview Diagram of All Therapeutic Targets in COVID-19
In a successful pharmaceutical development program, a good target must meet three essential criteria: efficacy, safety, and translational and commercial feasibility. This means that modulation of the target must demonstrate meaningful biological effects, avoid triggering off-target toxicity, and show relevance to clinical disease mechanisms. Additionally, the target should offer opportunities for developing molecules with favorable drug-like properties and differentiation in a competitive therapeutic landscape. To ensure these attributes, the process of target identification involves the integration of molecular mechanism exploration and systems biology. Strategies of target identification include:
However, identification alone is not sufficient. Rigorous target validation is required to experimentally confirm that modulating the candidate target produces the desired biological response. This step minimizes the risk of failure in later development stages and helps focus resources on the most promising opportunities. Key target validation approaches include:
MtoZ Biolabs combines these strategies into an adaptable target identification and validation framework that accommodates diverse biological systems, disease models, and therapeutic modalities. We help ensure that only robust, clinically actionable targets advance into the next phase of preclinical development.
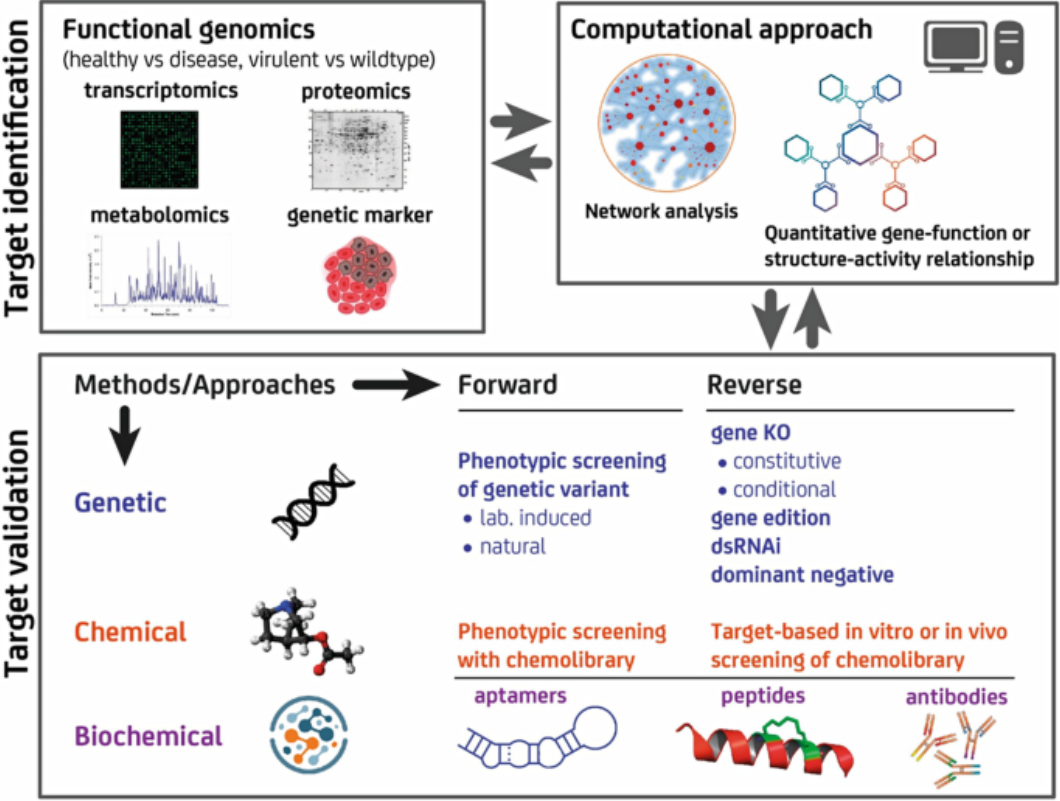
Figure 2. Overview of Methods for Drug Target Identification and Validation
Service Advantages
☑️Full-Process Coverage: From discovery to validation, we offer a seamless end-to-end workflow, reducing fragmentation and accelerating timelines.
☑️Multi-Omics Expertise: We integrate transcriptomics, proteomics, and metabolomics to identify biologically meaningful targets.
☑️High-Throughput Screening: Advanced CRISPR, RNAi, and compound libraries enable rapid functional validation in diverse models.
☑️Flexible Experimental Design: Customized solutions tailored to researchers' needs.
Sample Submission Suggestions
*Note: If you have specific requirements or need guidance on sample preparation, please do not hesitate to contact us.
Deliverables
1. Materials and Methods
Description of experimental design, sample types, and workflows used.
2. Key Instrument Settings
Instrument configurations and analytical parameters for reproducibility.
3. Raw and Processed Data Files
Includes raw MS files, FASTQ reads, and result tables as applicable.
4. Annotated Target List
Each target is annotated with supporting omics data.
5. Validation Summary Report
Functional data, pathway interpretation, and recommendations for next steps.
Related Services
High Throughput Drug Discovery Service
How to order?







