Sucrose Octaacetate Analysis Service | Pharmaceutical Excipient
Sucrose octaacetate (SOA) is an ester compound formed by the acetylation of the eight hydroxyl groups in a sucrose molecule. It is typically a white or off-white crystalline powder, characterized by an extremely strong bitter taste and high chemical stability. As a pharmaceutical excipient, it is commonly used to impart bitterness to drugs or as a taste-masking agent to improve patient compliance. It is also widely applied in formulation development to ensure the stability and consistency of drugs during storage and use. Its analysis generally relies on high-performance liquid chromatography (HPLC), mass spectrometry (MS), and related platforms to accurately determine its molecular structure, purity, and content.
Sucrose actaacetate analysis service based on pharmaceutical excipient has broad applications in the pharmaceutical industry. It can be used for quality control of taste-masking agents in tablets, capsules, and oral solutions, ensuring the stability of excipients during drug production and storage. In sustained-release formulation development, the properties of SOA contribute to regulating drug release rates and enhancing bioavailability. This service provides valuable support for further drug development.
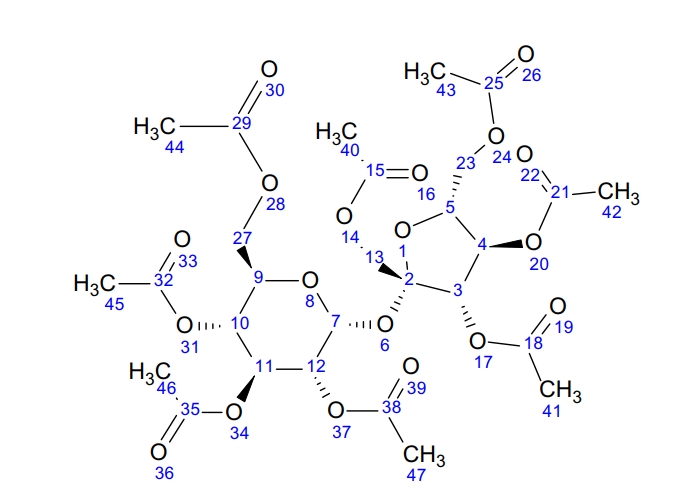
Stagner, W C. et al. Profiles of Drug Substances, Excipients and Related Methodology, 2019.
Figure 1. Numbering Scheme for Sucrose Octaacetate NMR Spectrum.
Services at MtoZ Biolabs
Based on advanced analytical platforms, MtoZ Biolabs has launched the sucrose octaacetate analysis service based on pharmaceutical excipient which enables comprehensive characterization and quality assessment of sucrose octaacetate. This service relies on high-performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) to precisely detect and quantitatively analyze the purity, content, structural features, and impurity components of sucrose octaacetate. The final data include its chemical composition, structural integrity, and distribution characteristics in different samples, providing reliable support for excipient quality control, stability studies, and compliance testing in pharmaceutical formulations.
Analysis Workflow
1. Sample Preparation
Samples are pretreated to remove impurities, ensuring the integrity and stability of sucrose octaacetate .
2. Separation and Purification
High-performance liquid chromatography (HPLC) is used to separate sucrose octaacetate, eliminating potential impurities and by-products.
3. Mass Spectrometry Analysis
High-resolution mass spectrometry (MS) is employed to detect the molecular weight and chemical composition of sucrose octaacetate, providing both qualitative and quantitative information.
4. Nuclear Magnetic Resonance (NMR) Analysis
NMR is applied to further analyze molecular structure and acetylation characteristics, confirming structural integrity.
5. Data Processing and Report Generation
Analytical results are integrated to generate a detailed report, including purity, content, structural features, and impurity distribution information.
Sample Submission Suggestions
1. Sample Type
Applicable to pharmaceutical excipient samples containing sucrose octaacetate. Samples can be in solid or liquid form, and the chemical integrity must be maintained.
2. Sample Purity
It is recommended to minimize impurities to improve analytical accuracy. For complex samples, preliminary purification is advised.
3. Sample Storage and Transportation
Samples should be stored and transported under light-protected and low-temperature conditions. Liquid samples should be kept in sealed containers, while solid samples must be tightly sealed and protected from moisture. Ice packs or dry ice may be used during transportation to ensure sample stability.
Service Advantages
1. High-Precision Analysis
Relying on advanced high-performance liquid chromatography (HPLC) and mass spectrometry (MS) platforms, sucrose octaacetate content, purity, and structural characteristics can be precisely detected, ensuring reliable data.
2. Multidimensional Detection
By combining multiple analytical techniques, information on the physicochemical properties, stability, and impurities of sucrose octaacetate can be obtained simultaneously, comprehensively evaluating its quality as a pharmaceutical excipient.
3. Customized Solutions
Based on customer research goals and sample characteristics, personalized testing and analysis strategies are provided, flexibly adapting to different application needs.
4. One-Stop Service
From sample pretreatment, detection to data interpretation and report generation, a complete one-stop solution is offered, simplifying experimental workflows and improving efficiency.
Applications
1. Drug Formulation Development
As a commonly used pharmaceutical excipient, sucrose octaacetate analysis helps optimize drug formulations, ensuring sustained release, taste-masking, and stability effects.
2. Quality Control and Testing
Sucrose octaacetate analysis service can detect the purity, content, and impurities of sucrose octaacetate, ensuring consistency and compliance in pharmaceutical production.
3. Food and Nutrition Research
In functional foods and nutritional products, analysis of sucrose octaacetate can be used for formulation standardization and quality monitoring, improving product safety and taste stability.
4. Industrial and Applied Research
Sucrose octaacetate analysis service can be used to evaluate the performance and stability of sucrose octaacetate in other industrial fields, such as applications in additives and materials science.
FAQ
Q1: Is Sample Pretreatment Required during the Analysis Process?
A1: Yes. Samples usually need to be dissolved, filtered, or extracted to remove impurities, ensuring the accuracy and reproducibility of the test results.
Q2: Why Does Sucrose Octaacetate Require Strict Testing as a Pharmaceutical Excipient?
A2: As a pharmaceutical excipient, sucrose octaacetate may affect drug stability, release rate, and taste-masking performance. Strict testing ensures consistency and compliance across different production batches.
How to order?







