Small Molecule Drug Screening Service-Ion Channels
Ion channels are made up of special proteins produced by cells, which are aggregated and embedded in the cell membrane, forming pores occupied by water molecules. These pores are the channels for water-soluble substances, which can quickly enter and exit the cell.
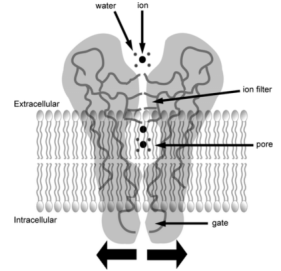
Figure 6. Schematic Diagram of Ion Channel Structure [6]
The activity of ion channels is the ability of cells to regulate the speed of corresponding substances entering and exiting cells through the opening and closing of ion channels, which is of great significance for the realization of various functions of cells. For example, in immune cells, divalent cations such as calcium, magnesium, and zinc play an important role as second messengers that regulate intracellular signaling pathways. Monovalent cations such as sodium and potassium mainly regulate membrane potential, indirectly controlling calcium influx and immune cell signaling.
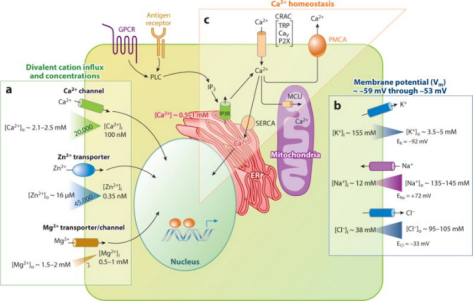
Figure 7. Regulation of Ion Channels and Divalent Cation Signaling in Immune Cells [7]
In the human genome, the pore-forming ion channel family is larger than the nuclear hormone receptor family, about half the kinase and protease family, and accounts for about 20% of the estimated 10,000 human protein-coding genes. The ion channel family is closely involved in almost all aspects of physiology and plays key roles in a variety of processes such as nerve and muscle relaxation, cognition, sensory transduction, blood pressure regulation, and cell proliferation. Their regulation has been implicated in a variety of conditions, including heart disease, neurological indications, kidney failure, pain perception, and blindness. More than 60 "channel diseases" have been identified as human diseases caused by ion channel mutations. Given the central functional role that the ion channel superfamily plays in human physiology, its membrane localization, and the diverse tissue distribution of different members of the family, it represents an attractive class of potential targets for drug discovery.
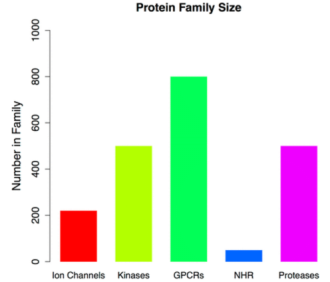
Figure 8. Relative Size of Ion Channels and Other Protein Families [8]
According to the different gating mechanisms, ion channels are divided into three categories: (1) Voltage gating, also known as voltage-dependent or voltage-sensitive ion channels: its open and close due to changes in membrane potential, named after ions that are easy to pass through, such as potassium, sodium, calcium, and chloride channels, each of which is divided into several subtypes. In excitable cells such as neurons, muscle cells, and glial cells, the switching of voltage-gated calcium channels is controlled by the membrane potential, which closes at resting potential, and opens when the membrane is depolarized, allowing calcium ions to enter the cell. There are several types of voltage-gated calcium channels: L-type calcium channels, P/Q-type calcium channels, N-type calcium channels, R-type calcium channels, and T-type calcium channels. The potassium ion channel is a transmembrane ion channel that allows potassium ions to selectively pass through the parapotential difference, and the potassium ion channel is composed of four subunits, forming a highly selective channel center pore. Potassium channel blockers can prevent potassium ions from passing through the channel holes. Their main function is to restore the cell to a resting membrane potential after an action potential. There are four types of potassium channels: calcium-activated potassium channels, inward-rectified potassium channels, Two‐pore domain potassium channels, and voltage-gated potassium channels. Sodium channels are transmembrane ion channels that selectively conduct sodium ions across the cell membrane. They are found in excitable cells, such as neurons and muscle cells, where they contribute to the influx of sodium cations and lead to a rising phase of action potential. There are two main categories: ligand-gated sodium channels and voltage-gated sodium channels. (2) Ligand-gating, alternately referred to as chemically gated ion channels. It is opened by the binding site on the transmitter protein receptor molecule, named after the transmitter receptor, such as acetylcholine receptor channels, glutamate receptor channels, aspartate receptor channels, etc. (3) Mechanical gating, also called mechanosensitive ion channels: it is a kind of channel that senses the stress change on the surface of the cell membrane and realizes the intracellular transduction of extracellular mechanical signals, which is divided into ion-selective and non-ion-selective channels according to permeability, and can be divided into tension-activated and tension-inactivated ion channels according to their functional effects. In addition, there are organelle ion channels, such as voltage-dependent anion channels widely distributed on the outer mitochondrial membrane of mammalian cells, receptor channels and receptor channels located in the organelle sarcoplasmic reticulum or endoplasmic reticulum, and membranes.
Advances in ion channel assay development have made it possible to screen for high-throughput screening (HTS) ion channel-related drugs.
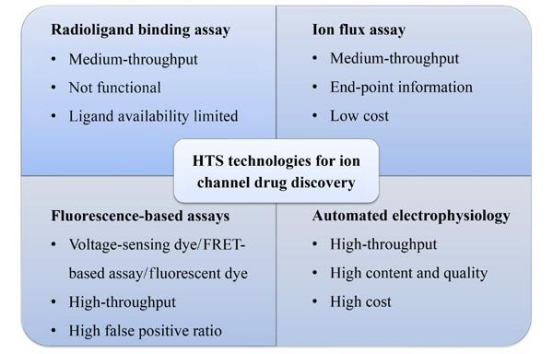
Figure 9. HTS Technology for Ion Channel Drug Discovery [8]
1. Radioligand Binding Assay
The radioligand binding assay uses a radiolabeled ligand, and when the radiolabeled ligand and unlabeled compound are incubated in a system, the compound will compete with the radiolabeled ligand if they can bind to the same site of the target protein. Thus, its IC50 value can be calculated based on the interference of the labeled ligand. This assay can detect the binding affinity of the drug using intact cells or isolated membranes, and there is a good correlation between the binding inhibition constant (Ki) and the functional test results (patch-clamp electrophysiology). There are also some drawbacks to this detection method, which involves a cleaning step during operation, making it difficult to achieve true HTS. Further testing is required to determine whether a candidate compound is an agonist or inhibitor.
2. Ion Flux Assay
Ion flux assays detect the efflux or influx of ions on the cell membrane by measuring the concentration of trace elements in extracellular solutions and cell lysates. Atomic absorption spectrometry (AAS)-based non-radioactive ion flux determination has been used since 1950s and has gradually replaced radioactive ion flux determination that relies on radiotracers and raises safety. AAS uses thermal energy to create atoms in the gas phase that absorb light at specific wavelengths. AAS assays have been applied to a variety of voltage-gated and ligand-gated channels, especially in potassium channels. In the classical experiments of this assay, Rb is used as a tracer ion of K to be loaded into cells expressing the target channel. After activating the channel by applying a ligand with a high K buffer (50-80 mM) or a depolarized membrane. AAS is uesd to sensitively quantify the concentration of tracer ions in supernatants and cell lysates. Ion channel activity is then analyzed by ion efflux or influx ratio. The assay achieves moderate throughput on 96- and 384-well plates. This throughput assay is currently widely used in the pharmaceutical industry.
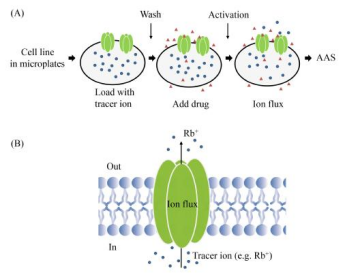
Figure 10. Schematic Diagram of Ion Flux Determination Using AAS [8]
3. Fluorescence-Based Assays
Fluorescence-based assays typically use CCD imaging devices to capture and detect these fluorescent signals for fluorescence measurements. Common dyes used in this process include ion-specific dyes and voltage-sensing dyes. Voltage-sensing dyes are tracers of voltage changes on the membrane. They are redistributed according to the depolarized or hyperpolarized state of the membrane. Oxyfusel derivatives, such as bis-(1,3-dibutylbarbituric acid) trimethoxy alcohol (DiBAC4), are negatively charged, voltage-sensing dyes that bind to the outer layer of the cell membrane in a quiescent or hyperpolarized state. When the cell membrane is depolarized, the dye moves to the inner layer of the cell membrane, increasing cytoplasmic fluorescence intensity. When an oxyenol probe is loaded into a cell expressing an ion channel, the oxyenol will be distributed internally and externally to achieve a distribution equilibrium, depending on the actual transmembrane potential. Non-cell-penetrating quenching buffers are often used to eliminate fluorescence from dyes on the outer layer of the cell membrane.
In a FRET-based assay, a pair of dyes is used to track changes in cell membrane potential. The most commonly used FRET dye pair is the oxanthrall DiSBAC2 as the FRET receptor and the coumarin-linked phospholipid CC2-DMPE as the FRET donor. In this assay, the FRET donor is a coumarin dye linked to a phospholipid inserted into the outer layer of the cell membrane, and the FRET acceptor is an oxanthrall derivative. At resting membrane potential (about -60 mV), the FRET donor and acceptor are in close proximity to each other in the outer layer of the cell membrane. The FRET donor is excited at 400 nm, emits a fluorescent signal of 460 nm, and the fluorescence energy is transferred from the donor to the oxanthrall acceptor, and finally emits a fluorescent signal at 580 nm. When the cell membrane is depolarized, the FRET receptor (oxyol) moves to the inner layer of the cell membrane, while the FRET donor remains in the outer layer of the cell membrane, resulting in a decrease in FRET emission at 580 nm and an increase in emission at 460 nm. The relative intensities of the 460 nm wavelength and the 580 nm wavelength were calculated as readings of the change in membrane potential. In a FRET-based assay, a coumarin donor is inserted into the cell membrane to specifically label changes in the voltage potential of the cell membrane rather than the organelle membrane.
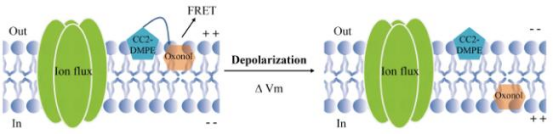
Figure 11. Schematic Diagram of FRET-Based Analysis [8]
4. Automated Patch-Clamp Electrophysiology (APC)
Traditional patch-clamp electrophysiology is a time-consuming task that requires highly skilled operation, which requires trained researchers to complete the experiments. The invention of automatic patch-clamp electrophysiology is a breakthrough in the realization of fully automatic processing of patch-clamp. APC has tested ion channel currents in a variety of cell lines, including CHO-hERG, HEK-ASIC3, CHO-Nav1.5, HEK-Nav1.2a, CHO-Kv.1.5, HEK-Nav1.7, and others. However, more physiological models are required to test ion channels. Cardiomyocytes and neurons from mouse or human induced pluripotent stem cells or embryonic stem cells have been recorded in the APC system, providing a deeper understanding of the physiological function of ion channels in vivo.
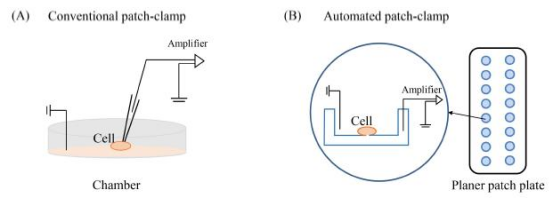
Figure 12. Schematic Diagram of Patch-Clamp[8]
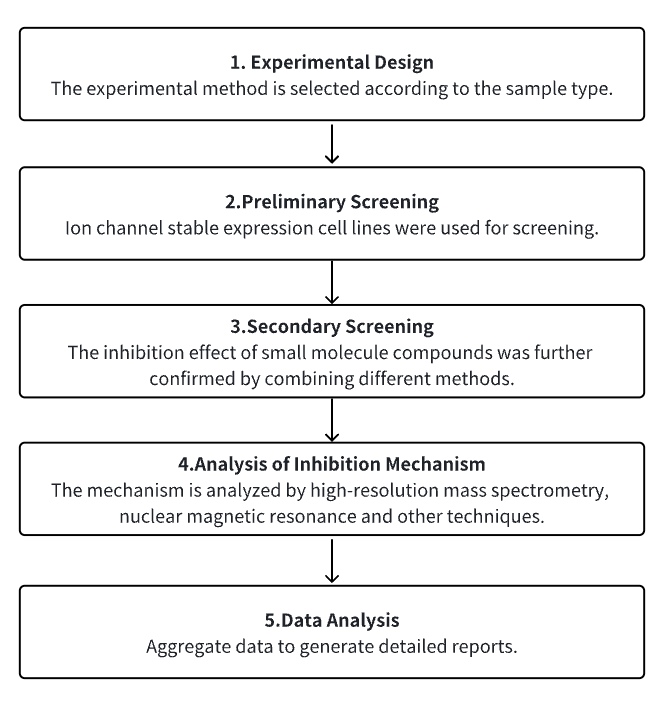
Analysis Workflow
1. Select the Experimental Method According to the Experimental Needs
2. Preliminary Screening of Cell Lines with Stable Expression of Ion Channels
3. Secondary Screening and Verification of Small Molecules with Research Potential
4. Analysis of Small Molecule Inhibition Mechanism
5. Data Analysis
Service Advantages
1. High-confidence screening of a variety of cell lines with stable expression of ion channels.
2. Validation of a variety of experimental methods (screening of voltage-gated or ligand-gated ion channels; functional testing of ion channels based on FLIPR; electrophysiology drug screening platform based on patch-clamp, etc.).
3. High confidence, high-precision mass spectrometry, and other technical analysis mechanisms.
4. Comprehensive bioinformatics analysis.
Sample Results
1. Comparative study of lysosomal cation channel TMEM175 using automated whole-cell patch-clamp, lysosomal patch-clamp, and electrophysiology of solid support membranes: functional characterization and HTS assay development. [9]
Lysosomal cation channel TMEM175 is a Parkinson's disease-associated protein and a promising drug target. Unlike whole-cell automated patch-clamp (APC), lysosomal patch-clamp (LPC) contributes to physiological conditions, but is not yet suitable for HTS applications. Here, solid-supported membrane electrophysiology (SSME) is studied, which can be both directly entered into lysosomes and high-throughput electrophysiological recordings. In SSME, a concentration gradient with a resting potential of 175 mV is used to stimulate TMEM0-mediated ion translocation. The concentration-dependent K response exhibits an I/C curve with two distinct slopes, indicating the presence of two conductive states. HTS assays for drug screening were developed and the SSME and APC assessment tool compounds (4-AP, zinc as inhibitors; DCPIB, arachidonic acid, SC-79 as enhancers) were used.
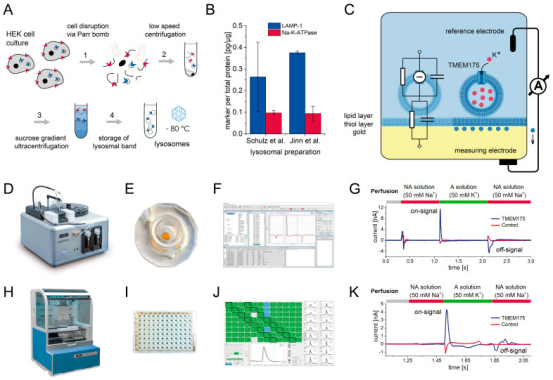
Figure 13. SSM-Based Electrophysiology Records the Principle of Localized TMEM175 in Lysosomes
2. 6-Iodoamiloride is found a potent acid-sensing ion channel inhibitor based on Automatic patch clamp (APC) screening. [10]
Acid-sensing ion channels (ASICs) are transmembrane sensors for extracellular acidosis and potential drug targets in several disease indications, including neuropathic pain and cancer metastasis. The potassium-sparing diuretic amiloride is a moderately nonspecific ASIC inhibitor that has been widely used as a probe to elucidate ASIC function. In this work, a library of 6-substituted and 5,6-disubstituted amiloride analogues was screened using a custom-developed automated patch-clamp protocol and 6-iodoamiloride was identified as a potent ASIC1 inhibitor.
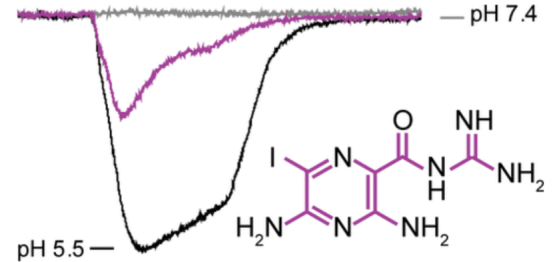
Figure 14. 6-Iodoamiloride Is A Potent Acid-Sensing Ion Channel Inhibitor
3. BAY-390, a selective central nervous system penetrating chemical probe, is discovered as an inhibitor of transient receptor potential ankyrin 1 (TRPA1). [11]
TRPA1 is voltage-dependent, and ligand-gated ion channels and their activation are associated with a variety of pain conditions. Preclinical studies have models demonstrating the role of the TRPA1 receptor in a wide range of animals for acute, inflammatory, and neuropathic pain. The study reported the identification of fragment-like hits from HTS activity and subsequent optimization to deliver a novel brain-penetrating agent TRPA1 inhibitor (compound 18, BAY-390), now available as an open source to the research community as an in vivo probe. Using the reactive agonist cinnamaldehyde (CA), 4 million compounds against human TRPA1 were screened at a concentration of 5 μM in a calcium fluorescence assay. The assay uses a 1536 microplate using a recombinant Chinese hamster ovary (CHO) cell line expressing hTRPA1 and a genetically encoded calcium sensor, GCaMP6, to measure hTRPA1-mediated calcium influx on a fluorescence imager. Given that the TRPA1 channel can be activated by a variety of reactive and non-reactive agonists, such as zinc 39 and PF-484015440, we further confirmed the activity of this target using zinc chloride as a non-reactive endogenous agonist.
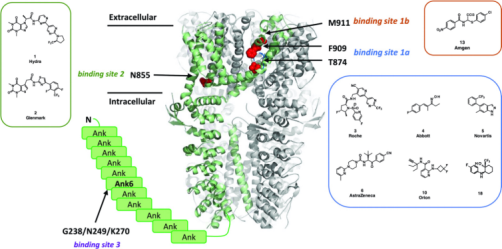
Figure 15. TRPA1 Antagonist Binding Site
4. The antihypertensive drug candesartan (CDST) can be used as a TMEM16A channel inhibitor. [12]
TMEM16A, a calcium-activated chloride channel (CaCC), and its pharmacological inhibitors inhibit the growth of lung adenocarcinoma cells. However, the poor efficacy, safety, and stability of TMEM16A inhibitors limit the development of these drugs. Therefore, finding new therapeutic directions from drugs that are already on the market is a feasible strategy to obtain safe and effective therapeutic drugs. The study screened a library of more than 2,400 FDA, EMA, and NMPA-approved drugs through virtual screening. A drug candidate CDST was identified, which showed strong inhibitory effects on TMEM16A in a concentration-dependent manner.
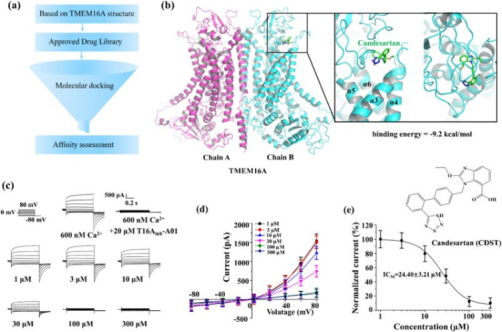
Figure 16. CDST Is A Novel TMEM16A Channel Inhibitor
Sample Submission Requirements
1. Provide pre-purified samples whenever possible.
2. Try to avoid impurity pollution.
Services at MtoZ Biolabs
1. Complete Experimental Protocol
2. Relevant Instrument Parameters
3. Raw Experimental Data
4. Detailed Information Report on Small Molecule Inhibitor Screening (including inhibition effect ranking, inhibition IC50, etc.)
Applications
1. Develop a new method for ion channel screening using bioinformatics analysis.
Computer-aided drug design (CADD) has been widely used to discover lead compounds with novel scaffolds with indicated targets from large chemical databases. Among these CADD methods, pharmacophore-based methods, molecular docking, and machine learning have been widely applied to discover novel chemistries that bind to specific targets. There are studies based on integrated virtual screening methods, biological evaluation, and molecular dynamics simulations to identify novel inhibitors targeting SGK1. In this study, a virtual screening platform for SGK1 inhibitors was established by ligand pharmacophore, structural pharmacophore, Bayesian classification, and molecular docking. The recovered molecules are then further filtered through the Five Rules (ROF), Veber's Rule, Pain, ADME/T properties, and various toxicities. Select and purchase potentially active compounds for enzyme activity testing. Finally, the binding mode, stability, and molecular interaction mode between the hit molecule and SGK1 under simulated physiological conditions were further explored through molecular dynamics simulations [13].
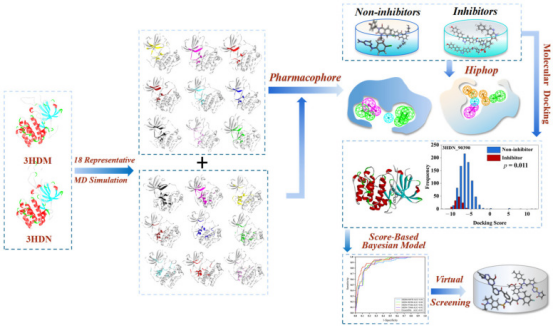
Figure 17. Discover Potent Inhibitors Targeting SGK1 Based on An Integrated Virtual Screening Strategy
2. Ion channel inhibitor group for the treatment of cancer[14].
Cancer is a complex disease. Currently, its main treatments are surgery, chemotherapy, radiotherapy, and biological therapy. The latter, including hormone therapy, growth-promoting tyrosine kinase inhibitors, and immunotherapies, is designed to activate the immune system to destroy tumors. While all of these approaches are effective, efficacy is often limited in time (the tumor gradually becomes resistant to treatment). In addition, adverse side effects are common, and these side effects can severely reduce quality of life. Therefore, in addition to constantly developing new treatment modalities, it is more convenient to make existing therapies more effective and convenient by combining them or in combination with other drugs. Studies have evaluated evidence on the effectiveness of combining conventional cancer treatments with ionic mechanism modulators, primarily channels that osmotic sodium, calcium, and potassium. The conclusion is that in each case, this combination can produce improved results by making a given treatment more effective and reducing adverse side effects. In addition, ion mechanism modulators themselves can exert anti-cancer effects.
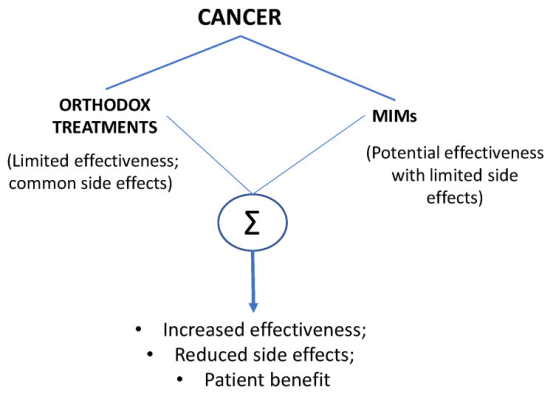
Figure 18. Comparative Advantages of Traditional Cancer Therapies Combined with Ionic Mechanism Modulators (MIMs)
FAQ
Q1: What are the fluorescent dyes for common ion channels?
Fluo-3 and Fluo-4 are fluorescent dyes widely used to measure intracellular calcium (Ga2+) changes. In the resting state, the concentration of intracellular calcium is about 10-7 mol/L, while the extracellular calcium is about 10-3 mol/L, which means intracellular Ga2+ concentration is 10,000 times lower than the extracellular Ga2+ concentration. Channel activation results in a large increase in intracellular Ga2+ concentration, which can be observed through detecting the alteration of Fluo-3/4 dye loaded into the cell. Therefore, calcium fluorescence indicators for calcium channels and non-selective cation channels have high SNR. There are also different ion-specific fluorescent dyes for K+, Na+, and Cl-, but these dyes are not widely used. Since only a small difference in the concentration of K+, Na+, and Cl- inside and outside cells, there is no drastic change when these channel are activated. However, the main disadvantage of fluorescence detection is the high rate of false positives, which requires further confirmation.
References
[1] Chan HCS, Shan H, Dahoun T, Vogel H, Yuan S. Advancing Drug Discovery via Artificial Intelligence. Trends Pharmacol Sci. 2019 Aug;40(8):592-604. doi: 10.1016/j.tips.2019.06.004. Epub 2019 Jul 15. Erratum in: Trends Pharmacol Sci. 2019 Oct;40(10):801. PMID: 31320117.
[2] Pinzi L, Rastelli G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int J Mol Sci. 2019 Sep 4;20(18):4331. doi: 10.3390/ijms20184331. PMID: 31487867; PMCID: PMC6769923.
[3] Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020 Mar 28;395(10229):1078-1088. doi: 10.1016/S0140-6736(20)30164-1. PMID: 32222192.
[4] Wacker D, Stevens RC, Roth BL. How Ligands Illuminate GPCR Molecular Pharmacology. Cell. 2017 Jul 27;170(3):414-427. doi: 10.1016/j.cell.2017.07.009. PMID: 28753422; PMCID: PMC5560499.
[5] Choi S, Choi KY. Screening-based approaches to identify small molecules that inhibit protein-protein interactions. Expert Opin Drug Discov. 2017 Mar;12(3):293-303. doi: 10.1080/17460441.2017.1280456. Epub 2017 Jan 20. PMID: 28067063.
[6] Bagal SK, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, Sidders B, Skerratt SE, Stevens EB, Storer RI, Swain NA. Ion channels as therapeutic targets: a drug discovery perspective. J Med Chem. 2013 Feb 14;56(3):593-624. doi: 10.1021/jm3011433. Epub 2012 Nov 29. PMID: 23121096.
[7] Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol. 2015;33:291-353. doi: 10.1146/annurev-immunol-032414-112212. PMID: 25861976; PMCID: PMC4822408.
[8] Guifang Duan, Bo Xu, Xia Yuan, Shuxiang Song. High-throughput screening technologies for ion channel drug discovery[J]. Journal of Chinese Pharmaceutical Sciences, 2021, 30(10): 785-793.
[9] Bazzone A, Barthmes M, George C, Brinkwirth N, Zerlotti R, Prinz V, Cole K, Friis S, Dickson A, Rice S, Lim J, Fern Toh M, Mohammadi M, Pau D, Stone DJ, Renger JJ, Fertig N. A Comparative Study on the Lysosomal Cation Channel TMEM175 Using Automated Whole-Cell Patch-Clamp, Lysosomal Patch-Clamp, and Solid Supported Membrane-Based Electrophysiology: Functional Characterization and High-Throughput Screening Assay Development. Int J Mol Sci. 2023 Aug 14;24(16):12788. doi: 10.3390/ijms241612788. PMID: 37628970; PMCID: PMC10454728.
[10] Finol-Urdaneta RK, McArthur JR, Aboelela A, Bujaroski RS, Majed H, Rangel A, Adams DJ, Ranson M, Kelso MJ, Buckley BJ. Automated Patch Clamp Screening of Amiloride and 5-N,N-Hexamethyleneamiloride Analogs Identifies 6-Iodoamiloride as a Potent Acid-Sensing Ion Channel Inhibitor. Mol Pharm. 2023 Jul 3;20(7):3367-3379. doi: 10.1021/acs.molpharmaceut.2c01083. Epub 2023 Jun 1. PMID: 37260417.
[11] Mesch S, Walter D, Laux-Biehlmann A, Basting D, Flanagan S, Miyatake Ondozabal H, Bäurle S, Pearson C, Jenkins J, Elves P, Hess S, Coelho AM, Rotgeri A, Bothe U, Nawaz S, Zollner TM, Steinmeyer A. Discovery of BAY-390, a Selective CNS Penetrant Chemical Probe as Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonist. J Med Chem. 2023 Jan 26;66(2):1583-1600. doi: 10.1021/acs.jmedchem.2c01830. Epub 2023 Jan 9. PMID: 36622903; PMCID: PMC9884088.
[12] Ji Q, Shi S, Ma B, Zhang W, An H, Guo S. Drug repurposing and molecular mechanisms of the antihypertensive drug candesartan as a TMEM16A channel inhibitor. Int J Biol Macromol. 2023 Apr 30;235:123839. doi: 10.1016/j.ijbiomac.2023.123839. Epub 2023 Feb 25. PMID: 36842737.
[13] Zhang H, Shen C, Zhang HR, Qi HZ, Hu ML, Luo QQ. Identification of Novel Inhibitors Targeting SGK1 via Ensemble-Based Virtual Screening Method, Biological Evaluation and Molecular Dynamics Simulation. Int J Mol Sci. 2022 Aug 3;23(15):8635. doi: 10.3390/ijms23158635. PMID: 35955763; PMCID: PMC9369041.
[14] Djamgoz MBA. Combinatorial Therapy of Cancer: Possible Advantages of Involving Modulators of Ionic Mechanisms. Cancers (Basel). 2022 May 30;14(11):2703. doi: 10.3390/cancers14112703. PMID: 35681682; PMCID: PMC9179511.
How to order?







