Recent Advances in PTMs Analysis Techniques: A Comparison of Mass Spectrometry and Other Methods
-
Multi-omics integration: PTMs data will be combined with transcriptomic, metabolomic, and other omics datasets to construct comprehensive regulatory networks.
-
AI-driven data interpretation: Advanced artificial intelligence algorithms will facilitate modification site prediction, noise reduction, and functional annotation, thereby improving the biological relevance and interpretability of results.
-
High-throughput and automated platforms: Automated mass spectrometry systems and streamlined sample preparation workflows will lower technical barriers and enhance consistency across datasets.
-
Spatial and single-cell resolution: By leveraging spatial omics and micro-proteomics techniques, in situ analysis of PTMs within tissue microenvironments will become increasingly feasible.
Protein post-translational modifications (PTMs) serve as essential regulators of cellular homeostasis, signal transduction, and gene expression. The rapid advancement of proteomics has positioned PTMs analysis at the forefront of biomedical research, offering valuable insights into disease mechanisms, biomarker discovery, and the development of targeted therapies. To achieve precise characterization of these modifications, researchers continue to refine analytical approaches, with mass spectrometry (MS) playing a central role and increasingly integrated with complementary emerging technologies. This review provides a systematic overview of the latest developments in PTMs analysis, highlighting key differences and similarities between MS-based and non-MS methods in terms of sensitivity, throughput, and applicability, to support informed decision-making in experimental design.
Why Are PTMs Important?
PTMs are widespread across biological kingdoms, including mammals, plants, and microorganisms. Common modifications such as phosphorylation, acetylation, methylation, ubiquitination, and glycosylation influence not only protein structural stability but also subcellular localization, molecular interactions, and degradation. In the context of complex diseases, PTMs often exhibit dynamic alterations that precede changes at the transcriptional or translational levels, thereby representing a more immediate and functionally relevant regulatory layer. As such, high-throughput, quantitative, and comprehensive PTMs analysis is pivotal in advancing modern life sciences.
Mass Spectrometry: The Core Platform for PTMs Research
Mass spectrometry (MS) has emerged as the cornerstone of PTMs characterization, owing to its superior resolution and quantification capabilities. Its strengths are reflected in the following aspects:
1. High-Throughput Detection
Leveraging enzymatic digestion, PTM-specific enrichment, and multi-stage MS acquisition, MS enables the simultaneous identification of thousands of modification sites in a single experiment—ideal for constructing global PTM maps.
2. Broad Modification Coverage
From well-characterized PTMs such as phosphorylation and acetylation to complex forms like glycosylation and polyubiquitination, MS supports the detection and quantification of diverse modifications through tailored protocols, facilitating the study of PTM interplay.
3. Precise Site Localization
MS provides amino-acid level resolution, allowing for precise localization of modification sites within protein sequences, thereby enhancing our understanding of how PTMs affect protein structure and function.
4. Dynamic Profiling Capability
When combined with quantitative analyses across experimental conditions, MS can capture PTM dynamics in response to stimuli, disease states, or drug treatments, offering insights into regulatory mechanisms at the post-translational level.
The Rise and Complementary Role of Other Methods in PTMs Analysis Techniques
While mass spectrometry holds a leading position in PTMs analysis, it also faces inherent limitations such as high instrumentation costs and complex data interpretation. Accordingly, several other non-mass spectrometry methods have demonstrated significant value in specific research contexts:
1. Antibody- or Probe-Based Detection
Platforms based on highly specific antibodies—such as immunoblotting, ELISA, and microarray technologies—enable rapid detection of known post-translational modifications, making them suitable for validation studies and high-throughput screening. However, their applicability is constrained by antibody quality and site specificity, which limits the discovery of novel modifications and reduces overall analytical coverage.
2. Chemical Labeling and Click Chemistry Approaches
Recent advances in chemical probe development, coupled with bioorthogonal “click chemistry” reactions, offer effective strategies for labeling and tracking specific PTMs. These methods are particularly advantageous in live-cell imaging and temporally resolved dynamic studies. Nonetheless, they are limited by the narrow range of detectable modifications and are sensitive to reaction conditions, posing challenges to broader application.
3. Single-Cell and Spatial Omics Applications
Driven by continuous technological progress, efforts have been made to extend PTMs analysis to single-cell and spatial omics resolutions. Although these approaches currently suffer from issues such as weak signal intensity and high background noise, they hold great promise for enabling high-precision studies of protein regulation and may shape the future landscape of functional proteomics.
Comparative Overview of Technical Choices
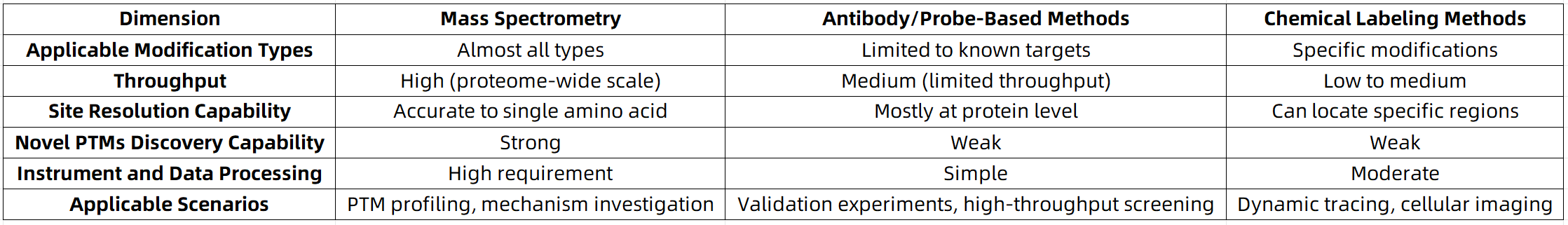
Future Trends: Integration and Intelligence Advancing in Parallel
PTMs analysis is evolving from isolated detection methods toward systematic and integrative approaches. Future developments are expected to exhibit the following key trends:
Recognized as the "regulatory code" of biological systems, protein post-translational modifications have gained substantial attention for their critical roles in cellular processes. With ongoing technological advancements—from high-resolution mass spectrometry to novel non-MS-based strategies—PTMs research is moving toward greater throughput, resolution, and functional insight. Researchers are encouraged to tailor their methodological choices based on experimental goals, sample types, and available resources. Partnering with a knowledgeable and well-equipped service provider can further accelerate the pace of discovery. MtoZ Biolabs offers professional, efficient, and customizable solutions for proteomics and PTMs analysis, supporting the detailed elucidation of molecular regulatory mechanisms.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







