Protein Complex Cryo-EM Structure Characterization Service
Protein complex Cryo-EM structure characterization is a high-resolution structural characterization technique based on Cryogenic Electron Microscopy (Cryo-EM), specifically designed to resolve the three-dimensional conformations of macromolecular protein complexes. This technique involves rapidly freezing samples under extremely low-temperature conditions without staining or crystallization, enabling the observation of the overall configuration, assembly state, and conformational dynamics of protein complexes in a near-native state. Through low-dose imaging and rapid cryo-fixation, this service maximally preserves the in situ structural state of proteins to achieve structural resolution.
Protein complex Cryo-EM structure characterization service is widely applied in research areas such as signal transduction complexes, transcription-translation complexes, membrane protein assemblies, antigen-antibody complexes, and drug targets. Structural characterization of protein complexes helps to elucidate protein-protein interaction interfaces, functional conformations, and ligand-binding sites, providing critical structural insights and mechanistic explanations for structural biology research, drug discovery, and protein engineering.
Services at MtoZ Biolabs
Based on a high-performance cryogenic electron microscopy system, the protein complex Cryo-EM structure characterization service provided by MtoZ Biolabs enables in-depth structural analysis of complex protein assemblies. This service preserves the in situ conformation of samples through rapid freezing and allows for unstained, high-resolution imaging of protein complexes. By integrating expert image processing and structural reconstruction workflows, it reveals the spatial configuration, subunit organization, and conformational heterogeneity of protein complexes, delivering three-dimensional reconstructions with high structural fidelity to support mechanistic studies and structure-function relationship analyses.
Analysis Workflow
1. Sample Preparation and Cryo-Fixation
After buffer optimization, protein complex samples are rapidly cooled to liquid nitrogen temperature to form vitreous ice, preserving their native conformation.
2. Low-Dose Image Acquisition
High-resolution imaging is performed in a cryo-electron microscope under low electron dose conditions to obtain high-quality two-dimensional micrographs.
3. Image Processing and Particle Selection
Acquired images undergo denoising, alignment, and automatic particle picking to extract target particles for structural reconstruction.
4. 2D/3D Classification and Reconstruction
Two-dimensional classification is first conducted to exclude aberrant particles, followed by three-dimensional reconstruction to resolve subunit architecture and the overall morphology of the complex.
5. Structural Annotation and Result Delivery
The resolved 3D structure is fitted with atomic models and functionally annotated, and a comprehensive analysis report is provided to support scientific publication and drug development.
Service Advantages
1. High-End Instrumentation Platform
MtoZ Biolabs is equipped with state-of-the-art cryo-EM systems (such as the Titan Krios), enabling high-resolution imaging and high-throughput data acquisition to ensure precision and efficiency in structural reconstruction.
2. Extensive Experience in Complex Analysis
We possess rich experience in resolving various types of protein complexes and are adept at handling high molecular weight samples, flexible regions, and conformational heterogeneity to improve the success rate of structure determination.
3. Flexible Adaptation for Diverse Complexes
Our services are tailored for a wide range of protein complexes—including membrane proteins, macromolecular assemblies, and dynamic systems—enhancing both sample compatibility and reconstruction efficiency.
4. One-Stop Structural Solution
From sample preparation and cryo-grid fabrication to image acquisition and 3D reconstruction, we provide a comprehensive, end-to-end service that covers the entire structural analysis workflow.
Applications
1. Multisubunit Protein Complex Structure Analysis
The protein complex Cryo-EM structure characterization service enables the elucidation of spatial organization and inter-subunit interactions within large macromolecular assemblies, supporting the understanding of functional mechanisms.
2. Signal Transduction Mechanism Studies
Applicable to investigating conformational changes and activation modes of key signaling complexes such as GPCRs, tyrosine kinases, and nuclear receptors.
3. Drug Target Binding Analysis
Allows visualization of binding patterns between small molecules, antibodies, or protein ligands and their targets, facilitating drug screening and structural optimization.
4. Flexible Conformational System Resolution
The protein complex Cryo-EM structure characterization service is suitable for analyzing protein complexes with multiple conformational states, including translational complexes, spliceosomes, and viral capsid assemblies.
Case Study
1. High-Resolution Cryo-EM of a Small Protein Complex: The Structure of the Human CDK-Activating Kinase
This study aims to explore the application of high-resolution cryogenic electron microscopy (cryo-EM) in structural characterization of small protein complexes, focusing on the human CDK-activating kinase (CAK) complex. Using advanced cryo-EM techniques, the research team determined the three-dimensional structures of CAK in its apo form, nucleotide-bound form, and in complex with 15 small-molecule inhibitors under non-crystalline conditions, achieving a maximum resolution of 1.8 Å. The results clearly revealed the internal conformations of the protein and the ligand-binding sites, as well as the water molecule networks in the active site that underpin inhibitor selectivity. The study demonstrates that cryo-EM can achieve near-atomic resolution even for small protein complexes, offering a viable approach and technical support for structure-based research in small-molecule drug development.
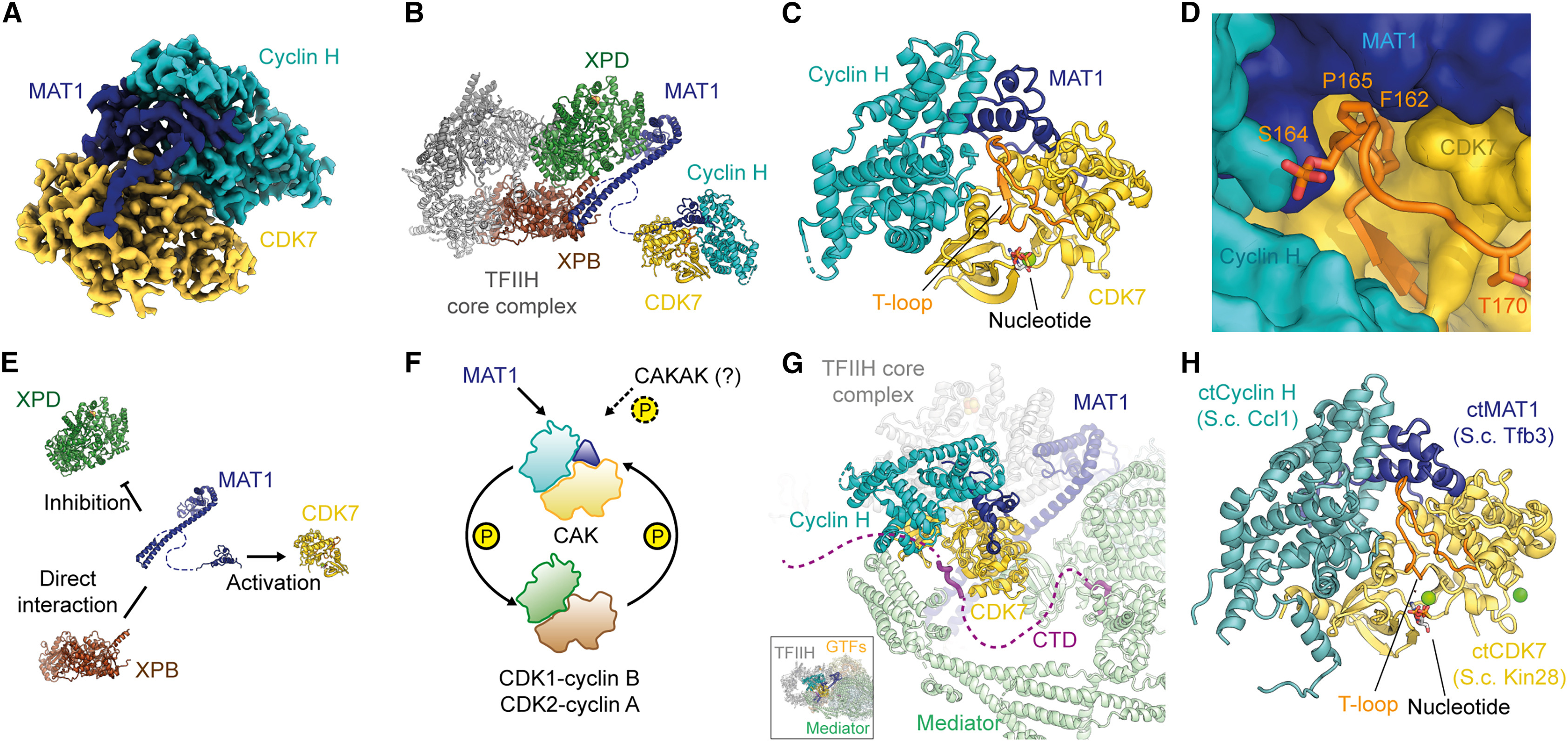
Greber, B J. Structure, 2024.
Figure 1. Cryo-EM Structure of the Human and Fungal CAKs.
2. Cryo-EM Structure of an Activated GPCR-G Protein Complex in Lipid Nanodiscs
This study aims to explore the application of high-resolution cryogenic electron microscopy (cryo-EM) in structural characterization of small protein complexes, focusing on the human CDK-activating kinase (CAK) complex. Using advanced cryo-EM techniques, the research team determined the three-dimensional structures of CAK in its apo form, nucleotide-bound form, and in complex with 15 small-molecule inhibitors under non-crystalline conditions, achieving a maximum resolution of 1.8 Å. The results clearly revealed the internal conformations of the protein and the ligand-binding sites, as well as the water molecule networks in the active site that underpin inhibitor selectivity. The study demonstrates that cryo-EM can achieve near-atomic resolution even for small protein complexes, offering a viable approach and technical support for structure-based research in small-molecule drug development.
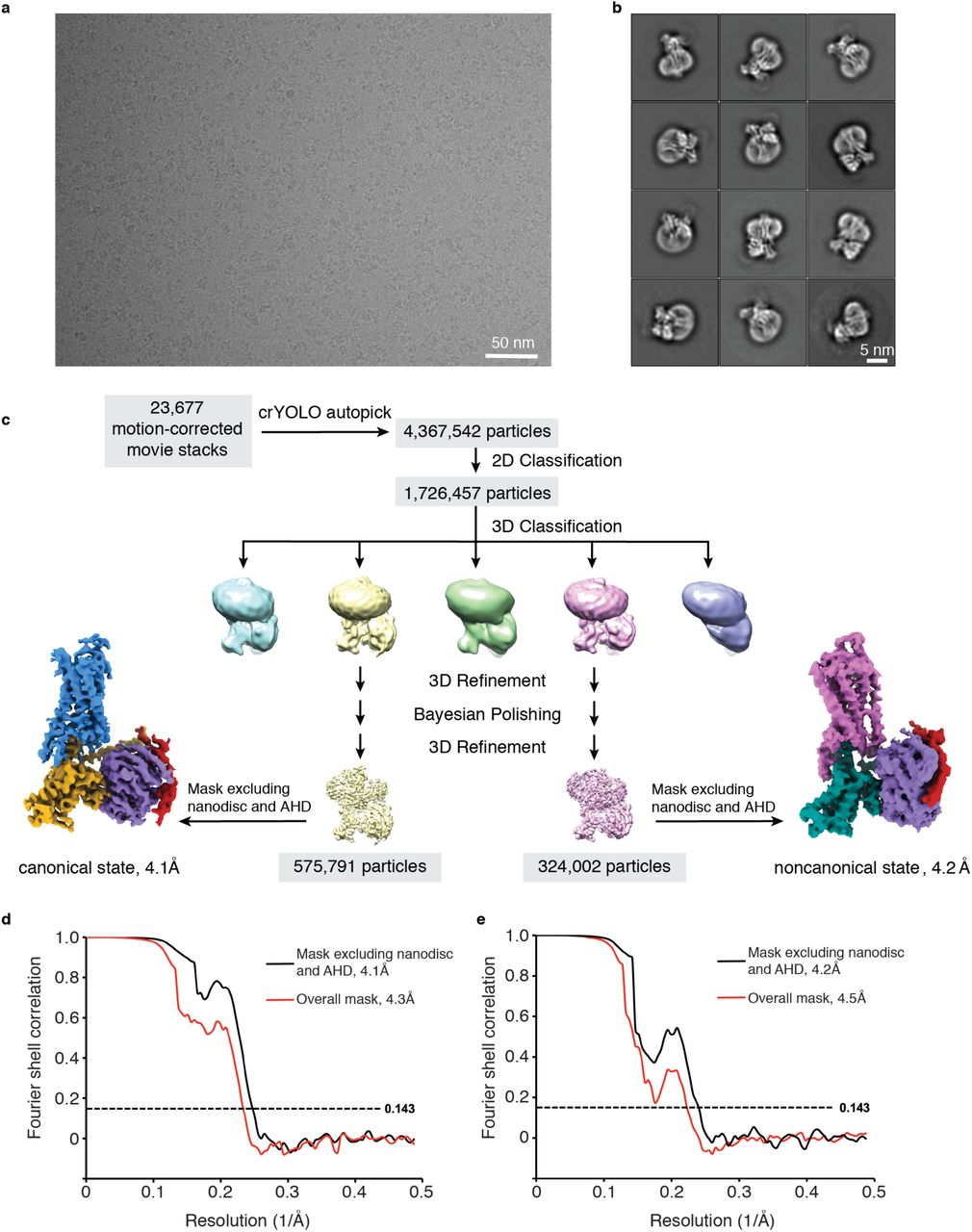
Zhang, M. et al. bioRxiv - Molecular Biology, 2020.
Figure 2. Cryo-EM Data Processing.
FAQ
Q1: What Types of Protein Complexes Are Suitable for Cryo-EM Structural Characterization?
A1: Cryo-EM is ideal for large molecular weight complexes (typically >150 kDa), multisubunit assemblies, highly flexible or crystallization-resistant protein complexes, including membrane protein complexes, signal transduction assemblies, and virus-like particles.
Q2: What Are the Advantages of Cryo-EM Compared to X-ray Crystallography?
A2: Cryo-EM does not require crystallization, allows visualization of dynamic conformations and flexible regions, is suitable for highly heterogeneous or non-crystallizable complexes, and generally requires a smaller amount of sample.
How to order?







