PRM vs. MRM: Comparative Analysis in Quantitative Proteomics
-
Quantification of known proteins in clinical samples using high-throughput analytical platforms
-
Targeted quantitative analysis during the biomarker validation stage
-
Investigation of drug mechanisms of action in pharmaceutical research
-
Validation of specific peptides in low-abundance samples with complex matrices
-
Confirmation of targets based on preliminary screening data generated through Data-Dependent Acquisition (DDA)
-
Studies requiring full fragment ion spectra to improve the reliability of peptide identification
-
For maximum sensitivity and high-throughput capability, MRM is the method of choice.
-
For enhanced specificity and efficient method development, PRM offers significant advantages.
-
In practice, the two approaches can be applied complementarily to establish a comprehensive quantitative workflow spanning from biomarker discovery to targeted validation.
In quantitative proteomics research, targeted quantification techniques play an increasingly critical role, spanning the workflow from target discovery to result validation. Compared to traditional approaches such as labeled quantification and data-dependent acquisition (DDA), Multiple Reaction Monitoring (MRM) and Parallel Reaction Monitoring (PRM) offer superior specificity, sensitivity, and reproducibility—making them especially advantageous for the precise detection of low-abundance proteins. With the advancement of high-resolution mass spectrometry, PRM has demonstrated growing potential to complement or even replace MRM in certain applications. This article provides a systematic comparison of PRM and MRM across dimensions including technical principles, performance characteristics, application contexts, and data analysis strategies, serving as a reference to guide methodological selection in quantitative proteomics.
Comparison of Technical Principles
1. Multiple Reaction Monitoring (MRM) is implemented on triple quadrupole mass spectrometers (QqQ) and achieves highly selective quantification by monitoring specific m/z transitions between precursor and product ions.
2. Parallel Reaction Monitoring (PRM), in contrast, leverages high-resolution and high-accuracy mass spectrometry platforms such as Orbitrap or Q-TOF to perform comprehensive scans of all product ions derived from target peptides, yielding extensive ion information.
In essence, MRM is characterized by targeted ion transition monitoring, whereas PRM provides full-spectrum ion acquisition, offering expanded analytical depth for targeted proteomics applications.
Performance Comparison: Sensitivity vs. Specificity vs. Throughput
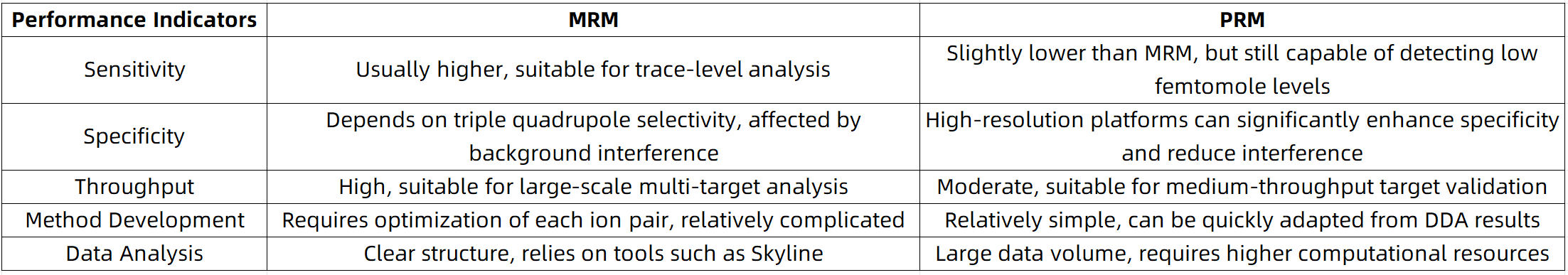
In terms of method development time, specificity, and data comprehensiveness, Parallel Reaction Monitoring (PRM) offers notable advantages in quantitative proteomics. However, Multiple Reaction Monitoring (MRM) remains the preferred technique when ultra-high throughput and maximal sensitivity are required.
Differences in Application Scenarios: From Large-Scale Screening to Precise Validation
1. MRM is particularly suitable for:
2. PRM is more appropriate for:
In targeted proteomics workflows such as cancer biomarker studies, PRM is commonly employed to validate candidate peptides identified in the DDA-based discovery stage. Subsequently, MRM enables large-scale, high-throughput quantification across broader clinical cohorts.
Data Processing and Software Support
Both PRM and MRM workflows are supported by open-source tools such as Skyline for method development and data analysis. However, differences in workflow structure and analytical complexity exist:
1. MRM focuses on predefined precursor–product ion transitions, generating structured datasets that are quick to process, making it ideal for standardized, high-throughput quantitative proteomics applications.
2. PRM involves extraction of targeted fragment ion signals from full MS/MS spectra, requiring spectral reconstruction and removal of interference peaks, which imposes greater demands on both computational algorithms and operator expertise.
Cost and Instrument Accessibility Considerations
1. MRM relies on triple quadrupole mass spectrometers, which are widely available and cost-effective to operate. As such, MRM is a routine quantitative platform in many hospitals and pharmaceutical laboratories.
2. PRM requires high-resolution instruments such as Orbitrap or quadrupole time-of-flight (Q-TOF) systems. These platforms entail higher capital and maintenance costs, making them more suitable for academic research institutions or advanced quantitative applications in targeted proteomics.
How to Choose Between PRM and MRM?
PRM and MRM each possess distinct strengths, and the choice depends primarily on the research objective and sample characteristics:
With extensive experience in operating mass spectrometry platforms and delivering targeted proteomics projects, MtoZ Biolabs has developed a robust and standardized PRM/MRM quantitative analysis pipeline. Our services accommodate diverse sample types—including plasma, urine, FFPE tissue, and fresh tissues, enabling customized targeted proteomics solutions. Feel free to contact us for tailored support in your quantitative proteomics research.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







