PRM and MRM in Targeted Proteomics: Advantages and Limitations
-
MRM: Requires extensive prior optimization of each transition and exhibits limited flexibility when applied to non-model organisms or post-translationally modified proteins.
-
PRM: The broader MS/MS scan range extends the cycle time, potentially reducing peptide coverage. Moreover, high-resolution mass spectrometers involve higher acquisition costs and demand more sophisticated operation and maintenance.
Targeted proteomics, recognized for its high quantitative accuracy and reproducibility, has emerged as a pivotal approach in biomarker validation, translational clinical research, and the investigation of drug mechanisms. At present, two primary mass spectrometry-based targeted strategies—Multiple Reaction Monitoring (MRM) and Parallel Reaction Monitoring (PRM)—have demonstrated distinct technical characteristics and application-specific strengths. This article provides an in-depth comparison of PRM and MRM in terms of fundamental principles, analytical performance, application contexts, and inherent limitations, aiming to guide researchers in selecting the optimal approach based on specific experimental objectives.
Overview of the Principles of MRM and PRM
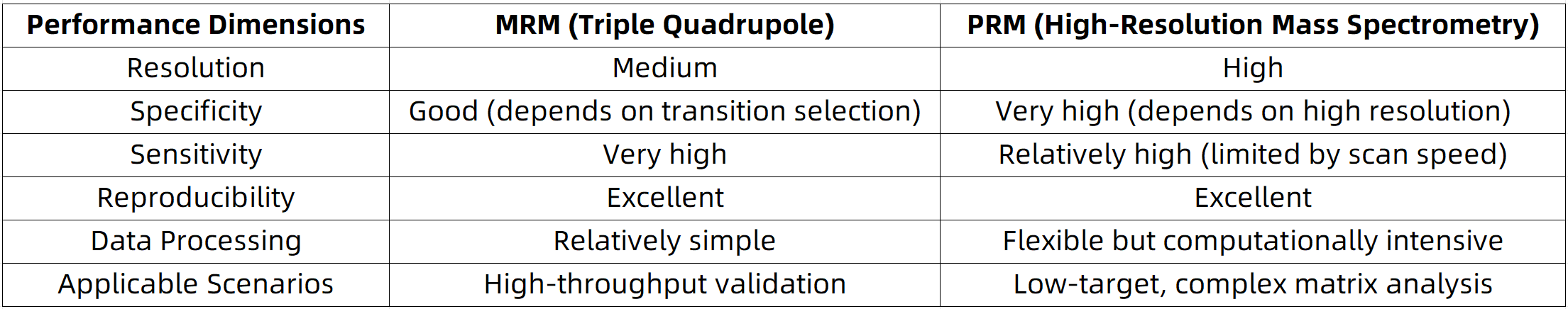
1. Multiple Reaction Monitoring (MRM)
MRM is typically performed on triple quadrupole (QqQ) mass spectrometers, where quantitative analysis is achieved by monitoring predefined precursor-to-fragment ion transitions. This method is characterized by rapid acquisition speed and high sensitivity, making it particularly suitable for the targeted quantification of known proteins or peptides.
2. Parallel Reaction Monitoring (PRM)
PRM is implemented on high-resolution mass spectrometry platforms such as Orbitrap or Q-TOF systems. Unlike MRM, PRM does not rely on predefined fragment ions; instead, it performs full MS/MS scans of the selected precursor ions, allowing simultaneous acquisition of all associated fragment ions. This enables enhanced selectivity and greater flexibility in downstream data processing.
Performance Comparison between MRM and PRM
Selection of Technology: Determined by Research Objectives and Sample Complexity
1. High-Throughput Validation and Large-Scale Clinical Studies
MRM, featuring superior throughput and a broad dynamic quantitative range, is well-suited for the development of multiplexed quantitative panels and is extensively utilized during the validation phase of candidate biomarkers.
2. Detection of Low-Abundance Proteins and Analysis of Complex Biological Matrices
PRM, characterized by high mass resolution, is particularly advantageous for the accurate quantification of trace proteins in challenging matrices such as plasma and cerebrospinal fluid. This makes it especially valuable in early-stage biomarker discovery and mechanistic investigations.
Considerations of Methodological Limitations
Despite the excellent performance of both MRM and PRM in targeted proteomics, each approach presents specific technical constraints:
As two principal strategies in targeted proteomics, MRM is generally preferred for high-throughput multi-target screening, while PRM is favored for high-specificity, high-resolution validation of targeted peptides. MtoZ Biolabs leverages advanced mass spectrometry platforms, including the Thermo Q Exactive™ and Sciex Triple Quad™, to offer comprehensive customized services encompassing target design, peptide synthesis, method development, and sample quantification. Whether for the precise quantification of individual targets or large-scale biomarker profiling, we provide adaptable and robust solutions tailored to experimental needs, ensuring high-quality and reproducible quantitative outcomes.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







