Phosphorylation Biomarker Specificity Validation Service
Phosphorylation Biomarker Specificity Validation Service ensures that phosphorylation markers are reliable and specific by confirming site-specific phosphorylation in biological samples. Phosphorylation biomarkers provide insights into cell signaling, disease mechanisms, and drug responses. They are particularly valuable in studying cancer, neurodegenerative diseases, metabolic disorders, and immune dysfunction. However, the low abundance, transient nature, and context-dependent regulation of phosphorylation events present significant challenges in biomarker discovery and validation.
Ensuring the specificity and accuracy of phosphorylation biomarkers is critical for their clinical and research applications. Common challenges in phosphorylation biomarker specificity validation include false positives, cross-reactivity, and non-specific binding, which can lead to unreliable results. Antibody-based methods, while widely used, may lack site specificity, failing to distinguish between closely related phosphorylation events or detect low-abundance phosphosites with high confidence. Moreover, phosphorylation dynamics and sample complexity further complicate biomarker validation, necessitating high-resolution, quantitative approaches to confirm phosphorylation status in a biologically relevant context.
Service at MtoZ Biolabs
MtoZ Biolabs provides comprehensive Phosphorylation Biomarker Specificity Validation Service to ensure the accuracy, reproducibility, and biological relevance of phosphorylation-based biomarkers. Our service integrates high-resolution mass spectrometry (DIA-MS, PRM/MRM), phosphopeptide enrichment (IMAC, TiO₂), and targeted biochemical assays (Western Blot, ELISA, and Proximity Ligation Assay) to validate phosphorylation events with high specificity. We offer customized validation strategies, assessing biomarker stability, site-specific phosphorylation, and assay reproducibility across different experimental conditions. Our advanced workflows address false positives, cross-reactivity, and non-specific binding, ensuring biomarker reliability for clinical research, diagnostic development, and therapeutic applications.
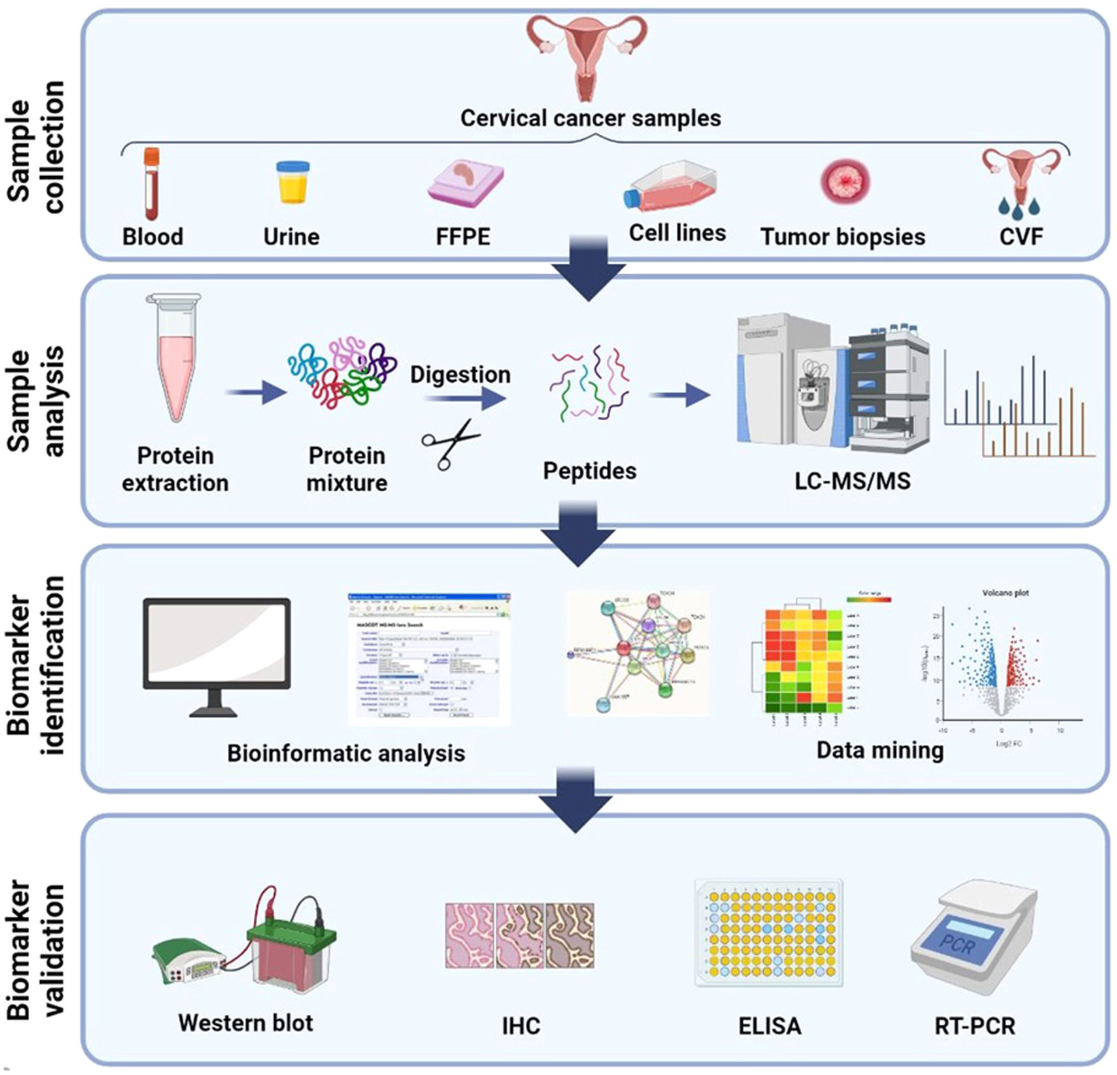
Figure 1. Schematic Illustration of General Method for Biomarker Validation of Cervical Cancer
Service Advantages
✔️High Sensitivity and Accuracy: Utilizes cutting-edge mass spectrometry and targeted assays to detect low-abundance phosphorylation events with high specificity and reproducibility.
✔️Comprehensive Multi-Platform Validation: Integrates phosphoproteomics, biochemical assays, and molecular biology techniques to ensure robust and multidimensional biomarker validation.
✔️Customizable Validation Strategies: Offers flexible experimental designs tailored to specific biomarker characteristics and research objectives, ensuring reliable and relevant validation.
✔️High-Throughput and Scalable Workflows: Leverages automated and high-throughput platforms to validate multiple phosphorylation biomarkers efficiently, accelerating discovery and clinical translation.
Applications
1. Clinical Biomarker Development
Phosphorylation Biomarker Specificity Validation Service ensures that phosphorylation-based biomarkers are accurately validated for clinical applications, supporting early disease detection, prognosis, and therapeutic monitoring.
2. Drug Target Identification and Validation
By confirming the specificity of phosphorylation events, Phosphorylation Biomarker Specificity Validation Service aids in identifying reliable kinase targets and phosphoproteins involved in disease progression, enhancing drug discovery and development.
3. Quality Control in High-Throughput Screening
Provides rigorous specificity validation in large-scale phosphoproteomics and biomarker discovery pipelines, ensuring high reproducibility and reliability across diverse experimental conditions.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
MtoZ Biolabs' Phosphorylation Biomarker Specificity Validation Service provide high-confidence validation, supporting biomarker translation into precision medicine and targeted therapies. Contact us to explore tailored solutions for your research.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Quantitative Phosphoproteomics Service
How to order?







