Pharmaceutical Solid-State Characterization Service
Pharmaceutical solid-state characterization is a key technique used to systematically evaluate the physical structure and thermal behavior of active pharmaceutical ingredients (APIs) and formulations in the solid state. This service integrates a variety of high-resolution thermal analysis and structural characterization methods to comprehensively assess the polymorphic forms, polymorphic transitions, thermal decomposition behaviors, and potential impurity information of APIs or formulations under different environmental conditions, thereby revealing stability changes and interaction mechanisms during storage, processing, or usage.
Pharmaceutical solid-state characterization service is widely applied in drug development, formulation screening, polymorph optimization, formulation process design, and quality control. Through solid-state characterization, it effectively guides the selection of polymorphic forms, evaluates excipient compatibility, monitors polymorphic changes during manufacturing, and enhances product stability and consistency. It plays an important role in the development of complex formulations and regulatory compliance for pharmaceutical products.
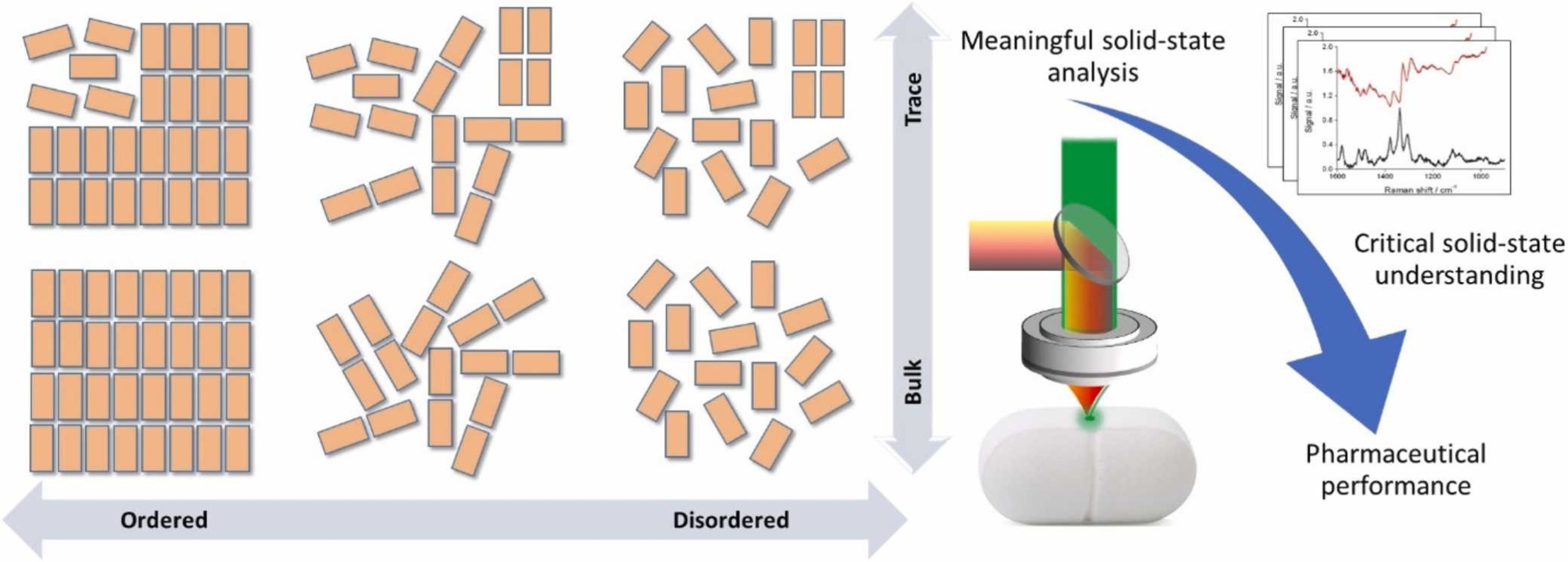
Rantane, J. et al. Journal of Pharmaceutical and Biomedical Analysis, 2023.
Figure 1. The Solid-State Analysis for Pharmaceuticals.
Services at MtoZ Biolabs
Based on high-resolution thermal analysis platforms and structural characterization instruments, MtoZ Biolabs offers pharmaceutical solid-state characterization service to comprehensively analyze the polymorphic forms, thermal decomposition behaviors, and phase transition processes of pharmaceutical solid-state samples. This service enables the assessment of the physical stability of APIs and formulations under various temperature and humidity conditions, providing key parameters such as decomposition temperature, polymorphic transition points, and gas release profiles. These data offer strong support for formulation development, manufacturing process optimization, and quality control. The analytical methods used for pharmaceutical solid-state characterization include but are not limited to the following:
1. EBSD (Electron Backscatter Diffraction)
Accurately reveals the grain orientation, grain boundary structure, and polycrystalline distribution of pharmaceutical crystals. It is suitable for studying the physical property differences and stability of various polymorphs.
2. TG-MS (Thermogravimetry–Mass Spectrometry)
Combines mass loss with gas molecular information to dynamically analyze decomposition pathways and the composition of volatile products during heating.
3. TG-IR (Thermogravimetry–Infrared Spectroscopy)
Monitors the infrared spectral features of gases released during pyrolysis in real time, used to identify decomposition products and validate impurity formation mechanisms.
4. In-situ XRD (In-situ X-ray Diffraction)
Tracks the evolution of pharmaceutical polymorphs under changing temperature or other stress conditions, such as crystallization, melting, polymorphic transitions, and amorphization.
Analysis Workflow
1. Sample Preparation
Appropriate pretreatment is performed based on the sample's physical state to ensure compatibility with subsequent testing requirements.
2. Method Selection
Characterization methods are selected according to the analytical objective, and testing parameters are configured accordingly.
3. Experimental Execution
Thermal analysis and structural monitoring experiments are carried out under predefined conditions, with real-time recording of the sample's thermal response and crystal structure changes during heating or isothermal processes.
4. Data Analysis
Key thermal parameters and structural information are extracted and cross-validated using multi-technique data to generate a comprehensive solid-state characterization report, supporting clients in formulation development and quality assessment.
Sample Submission Suggestions
1. Sample Type
It is recommended to provide purified solid drug samples, including powders, crystals, tablets, or lyophilized products. If the sample is in a special form (such as suspension or paste), please inform us in advance so that the testing feasibility can be evaluated.
2. Storage and Transportation
Samples should be sealed in clean and dry containers to avoid moisture, high temperature, and mechanical damage. Regular samples can be shipped at room temperature. For temperature-sensitive samples, low-temperature transportation with desiccant is recommended.
3. Additional Information
Please specify the sample origin, component information, intended testing purpose, and known physicochemical properties, so that the technical team can arrange the testing process appropriately and improve analytical accuracy.
Service Advantages
1. Multidimensional Characterization Capability
By integrating various solid-state analysis techniques, we comprehensively evaluate drug polymorphs, amorphous forms, and their transformation behaviors, revealing the correlations between solid-state properties and pharmaceutical performance.
2. High-Resolution Instrumentation Platform
Leveraging advanced instruments such as in situ XRD, TG-IR, TG-MS, and EBSD, we precisely capture microstructural details, thermal responses, and degradation pathways to enhance data reliability and interpretability.
3. Flexible Solution Customization
Customized analytical combinations are designed based on specific research stages and sample characteristics to meet diverse needs from raw material screening and formulation design to quality research.
4. Expert Technical Support
Our experienced solid-state characterization specialists provide one-on-one technical consultation and interpretation services to help clients identify potential risks and optimize product performance.
Applications
1. Formulation Development
Through solid-state form analysis, the optimal crystal form, polymorph, or amorphous state can be selected to improve drug solubility, bioavailability, and formulation performance.
2. Manufacturing Process Control
Pharmaceutical solid-state characterization service can be used to monitor changes in crystal form, particle size, and crystallographic orientation during production, ensuring consistency and quality stability in the formulation process.
3. Packaging Compatibility
By analyzing solid-state properties, the compatibility between the drug and packaging materials can be assessed to support the selection of appropriate packaging strategies and improve product storage and transportation safety.
4. Stability Study
Pharmaceutical solid-state characterization service can be used to evaluate crystal transformation, hydration, or dehydration behaviors under various storage conditions, helping to predict product stability throughout its lifecycle.
FAQ
Q1: Can this Service Be Used to Determine Transformations between Different Crystal Forms?
A1: Yes. Using in situ XRD, thermal analysis, and related techniques, we can monitor real-time crystal form transitions under conditions such as heat, humidity, or pressure, helping to assess stability and interconversion trends.
Q2: Can Trace Impurities or Mixed Crystal Forms Be Analyzed?
A2: Yes. We utilize high-sensitivity characterization platforms such as TG-MS, TG-IR, and simultaneous thermal analysis to detect low-level impurities and the coexistence of multiple polymorphs.
Q3: Do you Support Custom Project Design?
A3: Yes. MtoZ Biolabs can tailor testing protocols based on specific research goals such as polymorph screening, crystallization studies, or storage stability evaluation, by flexibly integrating various analytical techniques to meet project-specific needs.
Related Services
Electron Backscatter Diffraction (EBSD) Analytical Service
Thermogravimetric-Mass Spectrometry (TG-MS) Analytical Service
How to order?







