Payload Distribution & Encapsulation Efficiency Analysis Service | Cryo-EM
Payload distribution and encapsulation efficiency analysis refers to the comprehensive assessment of how therapeutic cargos—such as gene or small-molecule drugs—are packaged, retained, and spatially distributed within nanocarrier systems. In drug delivery platforms like lipid nanoparticles (LNPs), liposomes, virus-like particles (VLPs), and polymeric nanocarriers, payload molecules must be efficiently encapsulated within the carrier to ensure targeted delivery, protect the payload from degradation, and enable controlled release at the desired site of action. Encapsulation efficiency (%EE) quantitatively reflects the proportion of the input payload that is successfully incorporated into particles, while payload distribution analysis assesses the heterogeneity across particles—distinguishing between empty, partially loaded, and fully loaded particles on a single-particle basis.
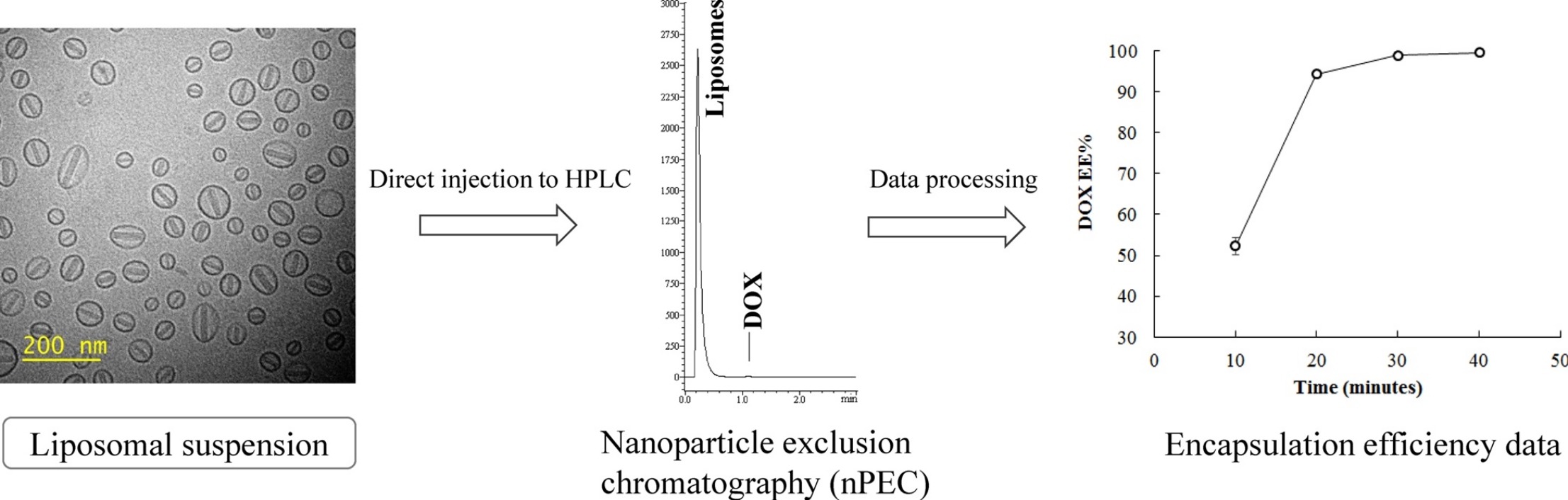
Figure 1. Liposome Encapsulation Efficiency Analysis
Accurate evaluation of payload distribution and encapsulation efficiency is critical for the development and quality control of nanoparticle-based drug delivery systems. Only when therapeutic cargos such as gene, proteins, or small molecules are efficiently and uniformly encapsulated can the intended biological effect be reliably achieved. A formulation containing a significant proportion of empty or partially loaded particles not only reduces therapeutic potency but also introduces variability in dosing and performance. This heterogeneity can affect safety, efficacy, and batch-to-batch consistency, posing challenges for both clinical translation and commercial production. Moreover, understanding how the payload is spatially distributed within individual particles provides valuable insights into the effectiveness of encapsulation strategies, the influence of formulation parameters, and the physical stability of the carrier under different storage or stress conditions.
While conventional bulk methods may provide overall loading percentages, they cannot reveal the structural integrity or encapsulation state of individual nanoparticles. In contrast, cryogenic transmission electron microscopy (Cryo-EM) enables direct, label-free visualization of each particle, allowing researchers to distinguish fully loaded particles from empty or compromised ones, and to correlate internal structural features with formulation performance. This particle-level analysis provides a far more detailed and meaningful assessment of encapsulation quality, supporting rational formulation optimization and high-confidence product development.
Service at MtoZ Biolabs
MtoZ Biolabs provides an advanced Cryo-EM-based Payload Distribution & Encapsulation Efficiency Analysis Service, enabling researchers to directly observe and quantify the encapsulation of nucleic acids, proteins, or drugs within a variety of nanocarriers. Through detailed image analysis, we classify particles based on internal electron density, providing accurate encapsulation efficiency metrics across the sample population.
Our Cryo-EM-based Payload Distribution & Encapsulation Efficiency Analysis Service platform supports a wide range of nanocarriers, including lipid nanoparticles (LNPs), virus-like particles (VLPs) such as AAV and capsids, drug-loaded liposomes, and polymeric nanoparticles. In addition to quantifying payload encapsulation, Cryo-EM also provides valuable structural information, allowing simultaneous evaluation of particle morphology, size uniformity, purity, and integrity. These insights further support comprehensive formulation assessment, enabling a deeper understanding of nanoparticle quality at both the single-particle and population levels.
Analysis Workflow
1. Sample Submission
Clients provide nanoparticle samples along with relevant formulation details such as particle type, concentration, and buffer conditions.
2. Pre-assessment & Grid Preparation
Samples are assessed for suitability and vitrified on EM grids using automated plunge-freezing to preserve native structure.
3. Cryo-EM Imaging
Multiple fields of view are acquired under low-dose conditions using high-resolution Cryo-TEM equipped with direct electron detectors.
4. Payload Analysis
Based on internal density features, particles are analyzed to evaluate the presence, distribution, and variability of encapsulated payload.
5. Data Processing & Quantification
Structural and encapsulation-related parameters are extracted to generate statistically meaningful insights across particle populations.
6. Report Delivery
A comprehensive report is delivered, including representative images, key quantitative results, and other detailed information.
Service Advantages
☑️End-to-End Cryo-EM Workflow: MtoZ Biolabs offers a fully integrated service—from sample vitrification and high-resolution imaging to expert classification and quantitative analysis—streamlining the entire evaluation process for clients.
☑️Customized Project Support: We tailor imaging parameters and analysis strategies to suit different nanocarrier types and payload characteristics, ensuring optimal data output for each formulation.
☑️High Flexibility and Collaboration: Whether you're in early R&D or preclinical development, we work closely with your team to define objectives, interpret data, and adapt to evolving project needs.
☑️One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Applications
1. Therapeutic Efficacy Evaluation
Distinguishing fully loaded from empty or underloaded particles helps correlate formulation characteristics with expected therapeutic output.
2. Dose Uniformity and Batch Consistency
Single-particle encapsulation profiling ensures homogeneity across formulations, reducing variability in clinical performance.
3. Formulation Development and Screening
Structural insight into payload distribution supports optimization of lipid ratios, solvent systems, and loading protocols.
4. Comparative Analysis for Process Changes
Enables quantitative comparison of encapsulation outcomes across formulation conditions, batches, or manufacturing modifications.
5. Stability and Degradation Studies
Visualization of payload retention over time aids in assessing shelf-life, formulation robustness, and storage condition effects.
Case Study
Morphology-Driven Optimization of Liposomal Drug Loading
A published cryo-TEM study investigated Doxil-like PEGylated liposomes encapsulating doxorubicin-sulfate crystals. As drug-to-lipid ratios increased, particles exhibited crystal-induced deformation from spherical to elongated forms. Cryo-TEM enabled single-particle analysis of payload content, liposome shape, and internal crystal length, revealing that excessive loading led to membrane rupture and formation of empty liposomes. The study concluded that Doxil’s original drug-to-lipid ratio is optimal for maximizing payload while minimizing structural distortion. This case highlights how cryo-TEM-based payload distribution and morphology analysis can guide rational formulation design, supporting the value of MtoZ Biolabs’ service in nanoparticle drug development.
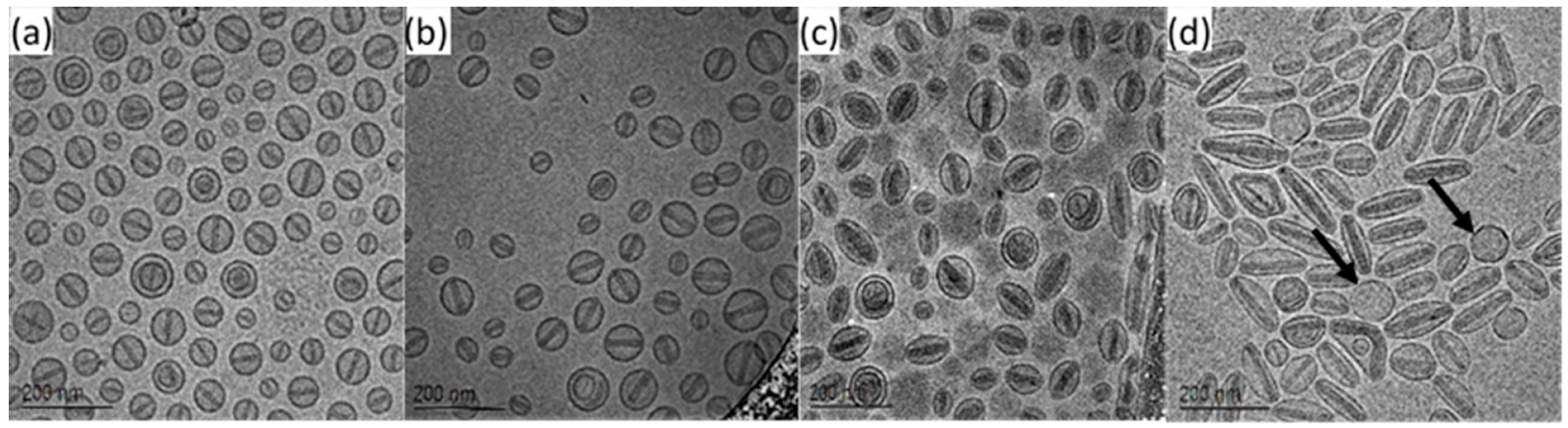
Figure 2. Liposome Elongatedness as a Function of Drug Loading: (a) 1 mg/mL, (b) 2 mg/mL, (c) 3 mg/mL and (d) 4 mg/mL—Black Arrows Point at Spherical Empty Liposomes
If you are interested in our Cryo-EM-based Payload Distribution & Encapsulation Efficiency Analysis Service, please feel free to contact us.
How to order?







