Oligo Mass Spectrometry Characterization Service
- Upon sample receipt, the type and analysis requirements (purity, sequence, impurity detection) of the Oligo are confirmed by our technical team;
- Pre-treatment methods (e.g., desalting, enzymatic digestion) are optimized via standardized protocols for modified or structurally complex Oligos (e.g., phosphorothioates).
- Chromatographic modes (IP-RP-LC, AEC, HILIC) are selected based on the physicochemical properties of the sample;
- High-capacity columns (e.g., C18, mixed-mode) are utilized to maximize separation efficiency.
- Accurate molecular weights and multistage fragment (MS²/MS³) data are acquired using high-resolution mass spectrometers (Q-TOF, Orbitrap);
- Resolution for complex samples (e.g., diastereomers) is enhanced through Ion Mobility Spectrometry (IMS) integration.
- Spectral interpretation, molecular weight distribution, and impurity profiles are generated by our expert analysis team, with complete sequence coverage reports;
- Key methodological validation parameters (sensitivity, reproducibility, LOQ/LOD) are rigorously documented in final reports.
- Solve complex sample separation challenges through 2D-LC integration;
- Suitable for analyzing long-chain oligos and highly modified samples.
- Led by a team of scientists with over 10 years of oligonucleotide analysis experience;
- Design customized experimental plans based on client needs (e.g., degradation acceleration stability studies).
Oligonucleotides (Oligo) are biomolecules composed of short chains of nucleotides and are widely used in gene therapy, cancer targeting, and rare disease treatments. As emerging therapeutic drugs, oligos exert their effects by regulating gene expression or directly targeting pathogenic RNA/DNA. However, their chemical synthesis complexity and structural heterogeneity pose strict requirements for quality control.
Anwar, S. et al. Pharmaceutics. 2023.
Figure 1. Some Commonly Used Oligo Chemistries
Key methods for Oligo characterization include liquid chromatography (HPLC/UPLC), mass spectrometry (MS), and capillary electrophoresis (CE). Among them, mass spectrometry (MS), with its high sensitivity and specificity, directly provides core data such as molecular weight, sequence information, modification sites, and impurity profiles. Through the multi-dimensional detection capabilities of liquid chromatography-mass spectrometry (LC-MS), it can address challenges related to sequence diversity, modification complexity, and accurately analyze key quality attributes such as phosphorothioate isomers, meeting the full-cycle quality control needs from R&D to production, ensuring the safety and efficacy of therapeutic oligos.
Vanhinsbergh, C. J. et. al. Anal Chem. 2022.
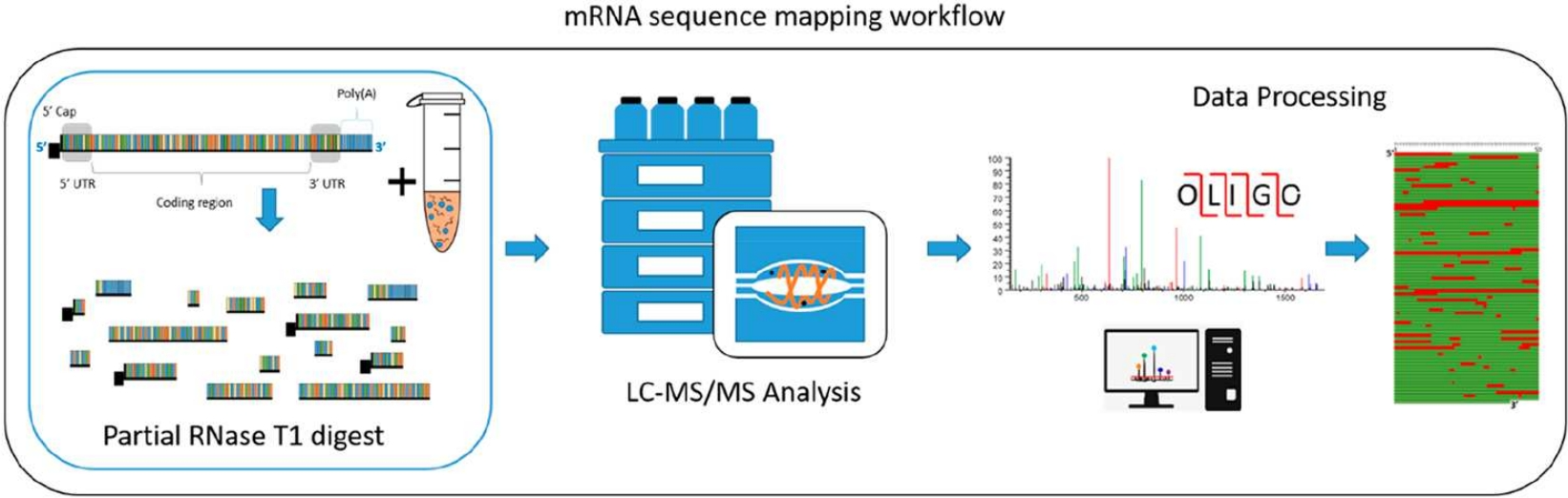
Figure 2. Schematic Illustration of mRNA Sequence Mapping Workflow
Services at MtoZ Biolabs
Based on advanced LC-MS platforms and industry experience, MtoZ Biolabs provides one-stop Oligo Mass Spectrometry Characterization Service for pharmaceutical R&D companies and research institutions, helping accelerate the translation process of oligonucleotide drugs from R&D to clinical applications. We offer full-dimensional characterization of oligonucleotides through high-resolution mass spectrometry (HRMS) and multi-dimensional chromatography (2D-LC-MS, HILIC-MS), covering:
1. Structural confirmation (molecular weight, sequence, modification sites)
2. Purity analysis (quantification of major components, identification of impurities and degradation products)
3. Modification analysis (distribution and homogeneity of modifications such as phosphorothioates, methylation, etc.).
We accept: antisense oligonucleotides (ASO), siRNA, CpG oligos, aptamers, and various chemically modified oligonucleotides (such as PS modifications, 2'-OMe, LNA, etc.).
Analysis Workflow
1. Sample Preparation and Assessment
2. Chromatographic Separation
3. Mass Spectrometry Detection and Data Acquisition
4. Data Analysis and Report Delivery
Why Choose MtoZ Biolabs?
1. High Resolution and Sensitivity
Equipped with Orbitrap Exploris 480 and SCIEX X500B Q-TOF mass spectrometry platforms, achieving detection limits as low as 0.1% impurities, meeting regulatory-level quality control requirements.
2. Multi-dimensional Technology Integration
3. Expert Team and Customizable Services
Applications
1. Oligo for Alzheimer's Disease Therapy
Hung, C. et al. Brain. 2024.
Figure 3. Oligo for Alzheimer's Disease Therapy
2. Oligo for the Treatment of Systemic Diseases of Liver Origin
Gogate, A. et al. Pharmacol Rev. 2023.
Figure 4. Oligo for the Treatment of Systemic Diseases of Liver Origin
Case Study
1. Separation and Characterization of Therapeutic Oligonucleotide Isomer Impurities by Cyclic Ion Mobility Mass Spectrometry
This study utilizes a cyclic ion mobility mass spectrometry (cyclic IMS) system to separate and characterize therapeutic oligonucleotide isomers. Therapeutic oligonucleotides, such as antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs), play an essential role in quality control, used to evaluate the quantities of target oligonucleotides and their impurities. Using cyclic IMS, the study successfully separated isomers of ASOs, including n-1 shortmers, abasic oligonucleotides, and PS→PO conversion isomers. These isomers exhibited distinct mobiligram patterns, demonstrating the ability of cyclic IMS to differentiate ions with the same m/z ratio based on structural differences. The results highlight the potential of cyclic IMS as a promising technique for evaluating therapeutic oligonucleotide isomers. Oligo Mass Spectrometry Characterization Service utilizes cyclic ion mobility spectrometry (IMS) to precisely separate and characterize therapeutic oligonucleotide isomers. This service effectively identifies and quantifies isomers with different structures, providing efficient solutions for quality control in therapeutic oligonucleotides. It supports the pharmaceutical and biopharmaceutical industries by ensuring the integrity and consistency of oligonucleotide-based therapies.
Omuro, S. et al. J Am Soc Mass Spectrom. 2024.
Figure 5. Mobiligrams of the Mixtures of n − 1 and Abasic Impurity Isomers of the Patisiran Sense Strand
2. Oligonucleotide mapping via mass spectrometry to enable comprehensive primary structure characterization of an mRNA vaccine against SARS-CoV-2
This study establishes an oligonucleotide mapping method based on LC-UV-MS/MS for the primary structure characterization of mRNA vaccines. Through single-enzyme digestion and high-resolution mass spectrometry, it evaluates the microheterogeneity of the 5′ cap structure and the 3′ poly(A) tail, enabling a thorough confirmation of the identity and integrity of mRNA products. This approach provides a crucial technical foundation for ensuring the quality and batch consistency of mRNA vaccines and can also be applied to broader RNA molecule structural analysis. Oligo Mass Spectrometry Characterization Service utilizes liquid chromatography and tandem mass spectrometry to deeply analyze the primary structure of mRNA and other RNA molecules. Our service also assesses 5′ and 3′ terminal modifications and microheterogeneity, supporting various RNA-related research and quality control requirements.
Gau, BC. et al. Sci Rep. 2023.
Figure 6. Batch Comparability and Construct Identity Assayed by Oligonucleotide Mapping
The future of oligonucleotide drugs relies on precise and efficient analytical techniques. With advanced mass spectrometry platforms, a strict quality system, and deep industry experience, MtoZ Biolabs is committed to becoming your core partner in the R&D process. Whether it is early-stage candidate screening or post-market batch quality control, we promise rapid response, reliable data, and compliance-free service to help overcome technical bottlenecks and shorten the drug development cycle. Contact us now for free technical consultation and personalized solutions!
How to order?







