O-glycosylation Analysis Service
- High-Resolution MS Platform: Leveraging advanced Orbitrap series instruments ensures superior fragmentation efficiency and high-quality spectra.
- Industry-Standard Glycan Database: Backed by a curated library of over 1,000 known O-glycans, we provide rapid annotation and dual-layer manual verification.
- Standardized Workflow: Typical turnaround time for routine samples is just 12–18 business days, with expedited service for urgent projects.
- Transparent Process: We offer interim data previews and flexible experimental adjustments.
- Publication-Ready Analyses: We provide in-depth support for study design, including journal-quality data visualization.
- Level Compliance: Adheres to international guidelines for glycosylation quality control in biological products.
- O-glycosylation Profiling for Therapeutic Proteins: Evaluate batch-to-batch consistency and process optimization.
- Risk Assessment of Unanticipated Glycosylation: Identify potential immunogenic sites in Fc-fusion proteins.
- Role of Aberrant O-Glycosylation in Tumors and Inflammatory Diseases: Uncover functional mechanisms.
- Biomarker Discovery and Clinical Validation: Support glycosylation-based biomarker screening in body fluids.
- Verification of Gene Editing Models (e.g., CRISPR Knockouts): Assess impacts on glycosylation pathways.
- Host Cell Protein (HCP) Analysis: Detect residual glycosylation modifications.
O-glycosylation is one of the most essential post-translational modifications governing protein function and regulation. It plays a pivotal role in disease mechanisms, immunotherapy, and the development of biopharmaceuticals. However, due to its high heterogeneity, dynamic modification profile, and the lack of universal analytical tools, achieving precise characterization remains a major challenge. Drawing on a mature glycosylation analysis platform, MtoZ Biolabs proudly offers its O-glycosylation analysis service, featuring high sensitivity, excellent reproducibility, and fully customized workflows. We provide reliable data support for academic research, clinical diagnostics, and the biopharmaceutical industry—accelerating scientific discovery and speeding drugs to market!
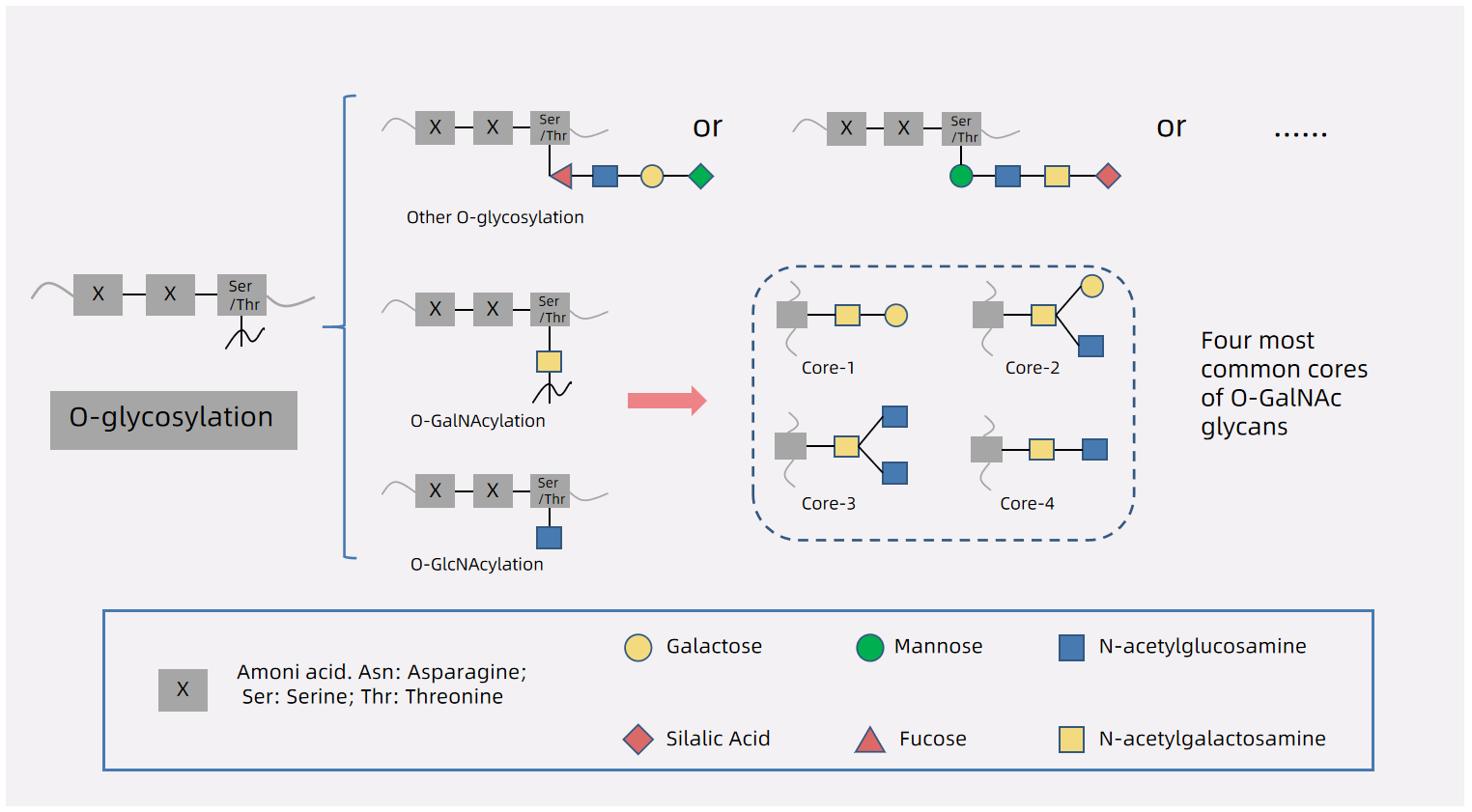
Figure 1. O-glycosylation Types of Glycoproteins
By integrating high-resolution liquid chromatography–tandem mass spectrometry (LC-MS/MS) with advanced collision dissociation technology, MtoZ Biolabs accurately pinpoints O-glycosylation sites and elucidates glycan structures:
1. Broad Sample Compatibility: Supports plasma, cell extracts, purified proteins (such as antibodies and fusion proteins), tissue samples, and pharmaceutical formulations.
2. High-Sensitivity Detection: Optimized glycopeptide enrichment enables precise capture of low-abundance O-glycosylation events.
3. Multi-Dimensional Structural Analysis: EThcD-based multi-fragmentation provides both glycan composition (including sialic acid, fucose, galactose, etc.) and site-specific information.
4. Flexible Analytical Approaches: Offers both qualitative (site/glycan) identification and semi-quantitative analysis to meet research exploration and quality control needs.
Kang, T. et al. Cell Rep Methods. 2024.
Figure 2. The Workflow of O-glycosylation Analysis
Why Choose MtoZ Biolabs?
1. Robust Technology, Reliable Data
2. Rapid Response, Fast Turnaround
3. Professional Service, Tailored Solutions
Case Study
1. Intact Mass Analysis Reveals the Novel O-linked Glycosylation on the Stalk Region of PD-1 Protein
Programmed cell death protein 1 (PD-1), a critical immune checkpoint receptor, contains a stalk region linking its extracellular and transmembrane domains. While widely studied structurally, its posttranslational modifications remain poorly characterized. This study reports novel O-linked glycosylation sites within PD-1’s stalk region (T153, S157, S159, T168) through O-protease digestion and intact mass spectrometry analysis. These residues were found to carry sialylated mucin-type O-glycans with core 1 and core 2 structures. The findings expand understanding of PD-1’s potential regulatory modifications and highlight an effective enzymatic-intact mass approach for O-glycosylation mapping, offering insights relevant to cancer immunotherapy. Our O-glycosylation analysis service employs targeted enzymatic digestion combined with advanced intact mass spectrometry to identify and characterize O-linked glycan sites on proteins. The service supports detection of sialylated mucin-type O-glycans, including core 1 and core 2 structures, enabling precise mapping of modification sites. Ideal for studying complex glycoproteins, it ensures high sensitivity in profiling glycosylation patterns without requiring prior glycan enrichment, providing crucial insights into protein posttranslational modification landscapes.
Tit-Oon, P. et al. Sci Rep. 2023.
Figure 3. Intact Mass Analysis of N-linked Removed Recombinant PD-1 Treated with Sialidases
2. Global O-glycoproteome Enrichment and Analysis Enabled by a Combinatorial Enzymatic Workflow
This study developed a combinatorial enzymatic workflow for global O-glycoproteome enrichment and analysis, addressing challenges in resolving O-glycosylation sites caused by the lack of consensus motifs, structural diversity, and absence of universal enzymatic cleavage tools. The strategy integrates N-glycan removal, combinatorial digestion using O-glycoprotease (IMPa) with proteases, glycopeptide enrichment, and stepped-collision-energy mass spectrometry. By analyzing human plasma samples, the workflow identified 189 O-glycosylation sites across 79 O-glycoproteins and 474 O-glycopeptides, revealing novel O-glycosylation sites on high-abundance proteins such as kininogen-1, fetuin-A, and apolipoprotein E. Independent validation experiments on purified proteins confirmed these newly identified sites, demonstrating the method’s capability to systematically decode O-glycosylation profiles in complex biological samples. O-glycosylation analysis service utilizes a multi-enzymatic workflow combined with advanced proteomic strategies to systematically characterize site-specific O-glycosylation. It integrates glycan removal, enzymatic digestion, and optimized enrichment methods for glycopeptide identification. Supported by high-resolution mass spectrometry, the service enables large-scale discovery of novel O-glycosylation sites across complex biological samples, including plasma and purified proteins, without dependence on prior sequence motifs. This approach ensures comprehensive mapping and validation of O-glycan modifications, facilitating exploration of understudied glycoproteomic landscapes.
Kang, T. et al. Cell Rep Methods. 2024.
Figure 4. Identification of O-glycosites and Their Glycan Compositions on Proteoglycan 4 (PRG4) Protein in Plasma Sample
Applications
1. Biopharmaceutical Development & Quality Control
2. Disease Mechanism & Biomarker Research
3. Basic Research & Translational Medicine
Deliverables
1. Comprehensive Site Map: Precise localization of all O-glycosylation sites in your sample, including modification frequencies.
2. Detailed Glycan Structures: Glycan compositions (hexoses, sialic acids, fucose, etc.), branching patterns, and modification profiles.
3. Comparative Analysis Report: Semi-quantitative profiling across multiple groups, uncovering key regulatory sites.
4. Bioinformatics Interpretation: KEGG/GO pathway enrichment to link glycosylation features with disease mechanisms or drug functions.
Whether you aim to publish groundbreaking research or secure approval for a critical therapeutic, MtoZ Biolabs is your most trusted partner. Our core strengths—solid technical expertise, efficient project execution, and flexible custom solutions—ensure cost-effective, high-value O-glycosylation analysis service for clients worldwide. Contact us to take advantage of our expert technical support and customized services tailored to your specific needs.
How to order?







