O-Glycan Modification and Site Analysis Services
- Protein: Minimum of 100 μg.
- Cells: At least 1x10⁷ cells.
- Animal Tissue: Approximately 1 g.
- Blood: 1 mL, must be anticoagulated with EDTA.
- Serum: Between 0.2 to 0.5 mL.
- Urine: At least 2 mL.
- Microbial Samples: 200 mg in dry weight.
O-Glycan Modification and Site Analysis is a specialized technology focused on characterizing the structural diversity, modification sites, and functional roles of O-glycans. O-glycans are a class of carbohydrate structures covalently attached to proteins via hydroxyl groups of serine (Ser) or threonine (Thr) residues, a process termed O-glycosylation. Unlike N-glycans, which follow a conserved Asn-X-Ser/Thr motif, O-glycans lack a fixed consensus sequence and exhibit remarkable structural diversity. The most common O-glycosylation type initiates with the addition of N-acetylgalactosamine (GalNAc) to Ser/Thr, forming the basis for eight core structures that are further extended with sugars like galactose, N-acetylglucosamine (GlcNAc), fucose, and sialic acid. This diversity enables O-glycans to regulate critical biological processes, including protein stability, cellular signaling, immune responses, and disease progression.
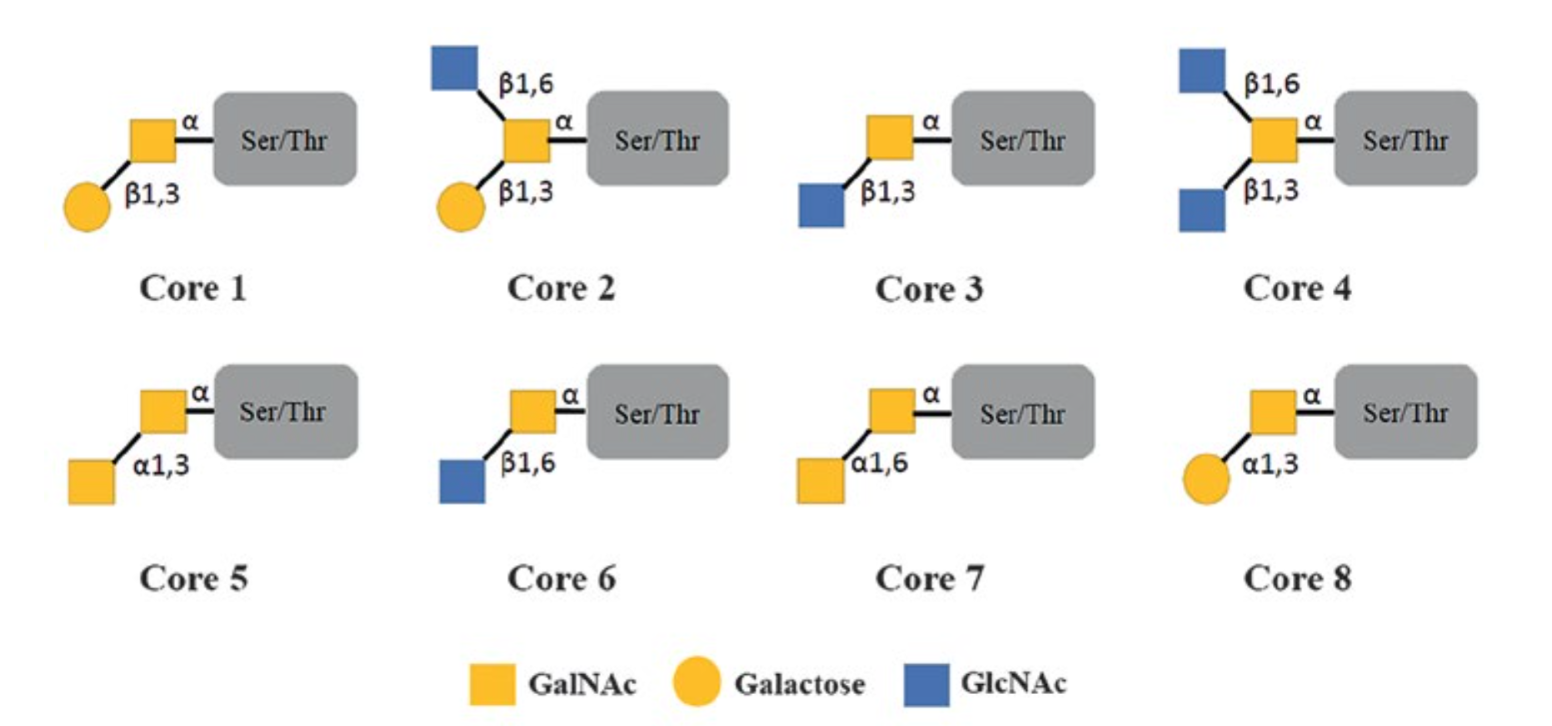
Figure 1. O-Glycan Structures Core 1-8
O-Glycan Modification and Site Analysis is pivotal to both physiological and pathological states. Aberrant O-glycan profiles are linked to cancer metastasis, autoimmune disorders, and neurodegenerative diseases. For instance, truncated O-glycans (e.g., Tn and sialyl-Tn antigens) serve as biomarkers for early cancer detection, while altered O-GlcNAcylation in cytosolic proteins modulates transcription and metabolic pathways.
Service at MtoZ Biolabs
MtoZ Biolabs provides O-Glycan Modification and Site Analysis Services to help researchers decode the complexity of protein O-glycosylation. Our platform combines O-glycoprotein/peptide enrichment, O-glycan release, structural characterization, and quantitative MS-based analysis to resolve O-glycan heterogeneity, define site-specific glycosylation, and link glycan profiles to functional outcomes. These capabilities support biotherapeutic development, biomarker discovery, and functional glycomics by revealing glycosylation-driven mechanisms in health and disease. To accommodate both focused and large-scale studies, we offer two analytical approaches:
1. Target Protein O-Glycan Modification and Site Analysis
For protein-specific investigations, MtoZ Biolabs delivers targeted characterization of O-glycan modifications on individual proteins. Using selective enrichment and high-resolution LC-MS/MS, we determine site occupancy, define glycoform structures, and quantify condition-dependent changes, supporting mechanistic evaluation and therapeutic protein assessment.
2. Proteome-Level O-Glycan Modification and Site Analysis
For broader discovery, our proteome-level workflow enables systematic profiling of O-glycosylation across complex biological samples. High-throughput LC-MS/MS acquisition, combined with specialized glycoproteomics pipelines, reveals global O-glycan diversity, abundance patterns, and network-level regulatory effects, offering comprehensive insights into O-glycosylation dynamics.
Analysis Workflow
1. Extraction and purification of proteins
2. Digestion of proteins
3. Enrichment and elution of peptides
4. Analysis by mass spectrometry
5. Interpretation of data
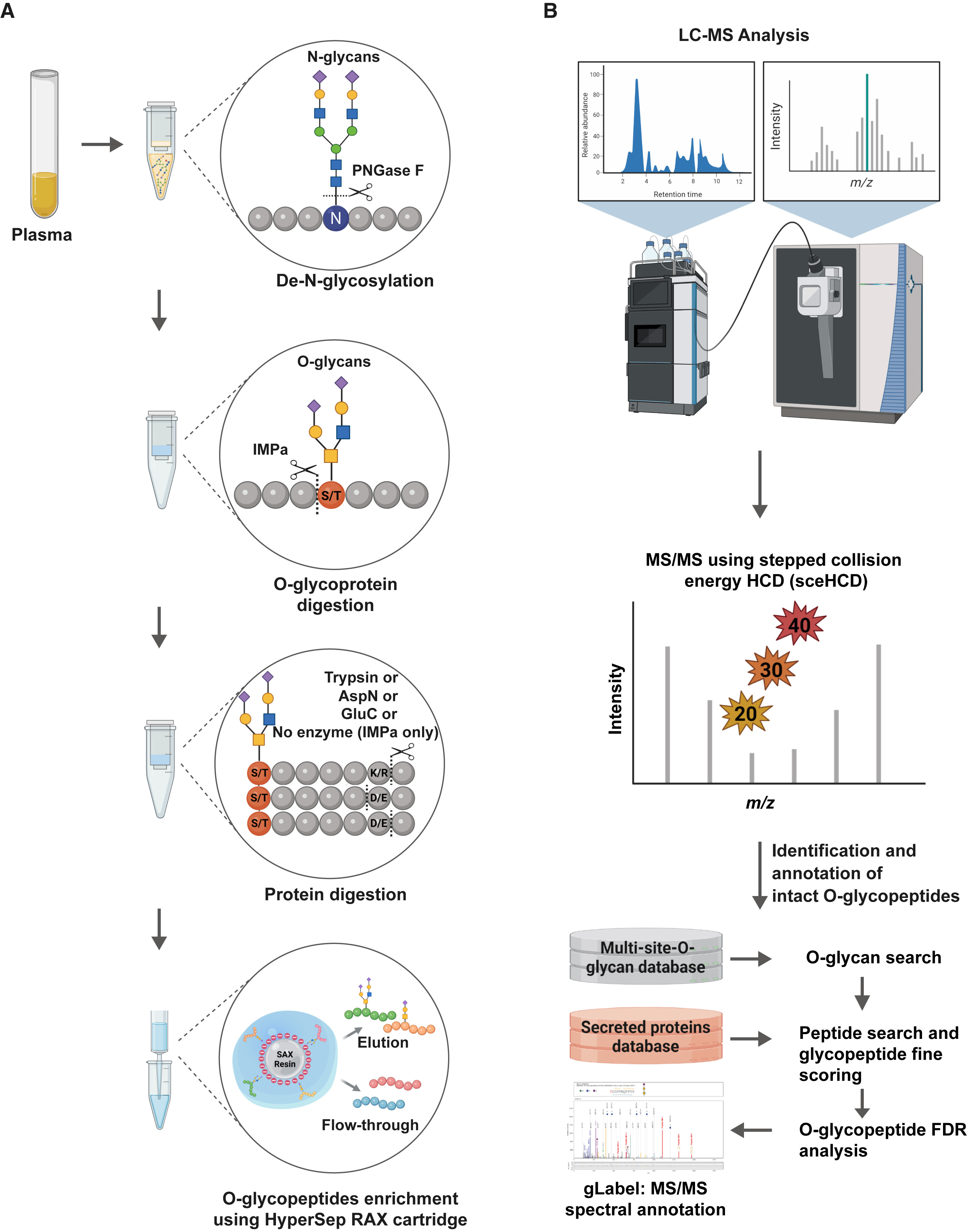
Figure 2. A Schematic Workflow Depicting Combinatorial Enzymatic O-Glycoproteomics
Service Advantages
✅Advanced Mass Spectrometry Platform: High-resolution mass spectrometry platform for precise glycan and site identification.
✅Experienced Expert Team: Seasoned scientists ensure accurate data interpretation and troubleshooting.
✅End-to-End Solutions: Full workflow from sample prep to bioinformatics, saving your time and efforts.
✅Broad Sample Adaptability: Compatible with tissues, serum, exosomes, disease-specific specimens, and more sample types.
Applications
1. Disease Mechanism Research
O-Glycan Modification and Site Analysis Services enable precise mapping of aberrant O-GlcNAc/O-GalNAc patterns in cancer, diabetes-associated glycoproteins, and neurodegenerative proteins like Tau. These services identify disease-specific glycosylation shifts, such as sialylation changes in tumor microenvironments.
2. Plant Science and Agriculture
O-Glycan Modification and Site Analysis Services decode stress-responsive glycosylation in crops. They also study O-GlcNAc-phosphorylation crosstalk in plant stress adaptation.
3. Therapeutic Protein Characterization
O-Glycan Modification and Site Analysis Services optimize antibody drugs by profiling O-GalNAc heterogeneity (e.g., PD-1/PD-L1 inhibitors). Clinical biomarker validation leverages serum/exosome O-glycome analysis for early cancer detection.
4. Basic Glycomics Exploration
O-Glycan Modification and Site Analysis Services unravel crosstalk between O-GlcNAc and phosphorylation. They also map glycosylation-driven protein conformational changes.
Case Study
O-Glycan Modification and Site Analysis in Epithelial Formation
In a study focused on understanding the role of mucin-type O-glycosylation in human health, researchers analyzed the isoform-specific targets of 20 N-acetylgalactosaminyltransferases (GalNAc-Ts) expressed in a tissue-forming human skin cell line. The study revealed significant biological effects of site-specific O-glycosylation on epithelial formation, with over 300 unique glycosylation sites identified across a diverse set of proteins, each regulated by specific GalNAc-T isoforms. A notable finding from the study was the high variability in O-glycosylation site occupancy across 70 glycosylated regions of secreted proteins. This insight underscores the functional significance of individual O-glycosylation sites in the proteome, demonstrating their impact on tissue phenotypes and cellular functions. This study exemplifies how O-Glycan Modification and Site Analysis Services can identify and quantify site-specific O-glycosylation patterns, offering valuable insights into glycosylation-driven biological functions, disease mechanisms, and therapeutic targets. Our advanced mass spectrometry-based services enable researchers to gain a comprehensive understanding of O-glycan modifications, facilitating biomedical research and biopharmaceutical applications.
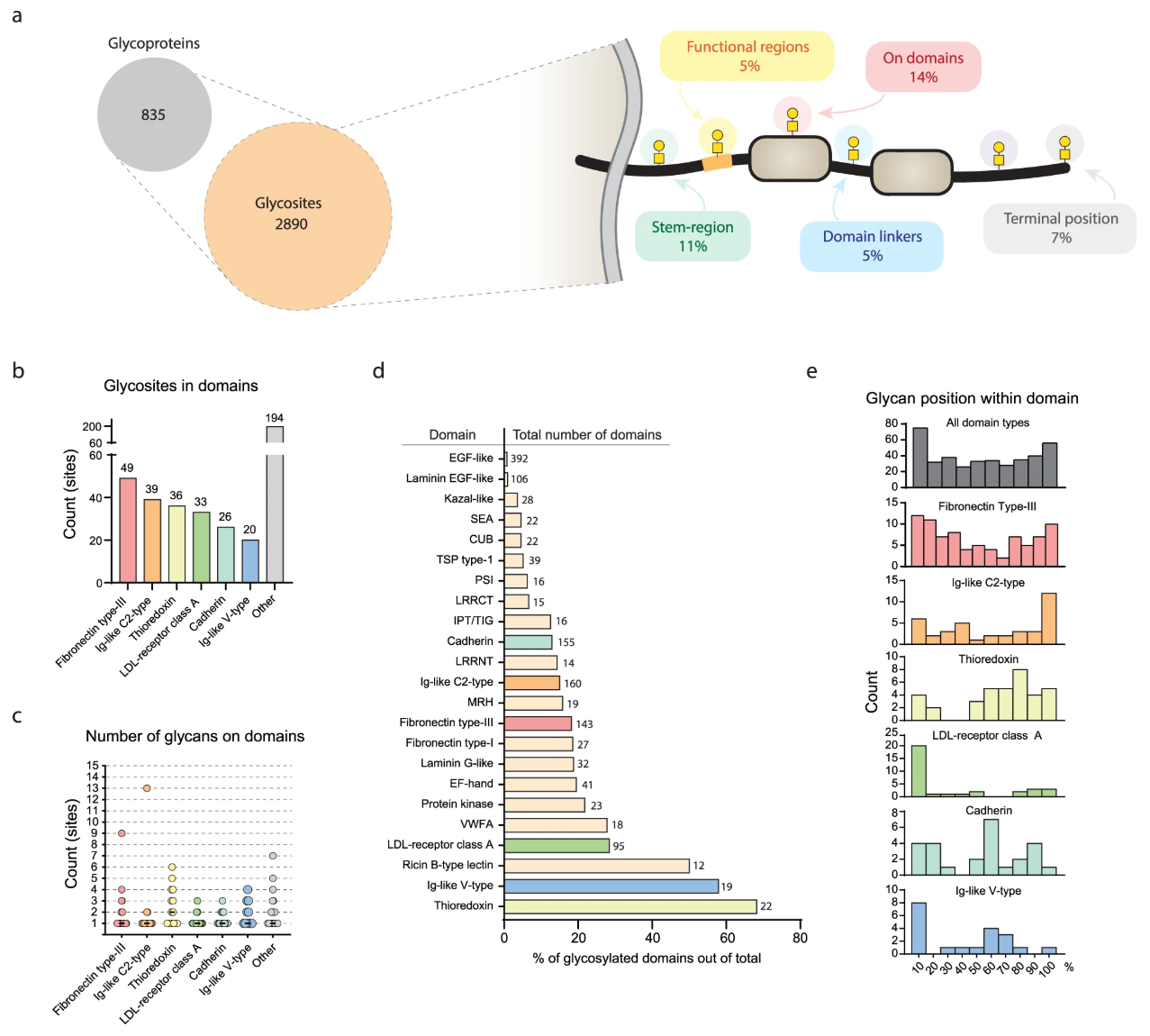
Nielsen, M. I. et al. Nat Commun. 2022.
Figure 3. O-GalNAc Glycans on Protein Domains
Sample Submission Suggestions
*Note: Should you have any inquiries regarding sample submission, do not hesitate to reach out. Our expert team is available to assist you at all times.
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on O-Glycan Modification and Site Analysis
4. Mass Spectrometry Images
5. Raw Data
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







