N-Glycan Modification and Site Analysis Services
- Protein: Minimum of 100 μg.
- Cells: At least 1x107 cells.
- Animal Tissue: Approximately 1 g.
- Blood: 1 mL, which must be anticoagulated with EDTA.
- Serum: Between 0.2 to 0.5 mL.
- Urine: At least 2 mL.
- Microbial Samples: 200 mg in dry weight.
N-glycans are complex carbohydrate structures covalently linked to asparagine (Asn) residues at the Asn-X-Ser/Thr motif, playing a crucial role in protein folding, stability, trafficking, and activity. As a key post-translational modification (PTM), N-glycosylation influences various biological processes, and its dysregulation is associated with diseases such as cancer, neurodegenerative disorders, autoimmune diseases, and metabolic syndromes, making it a critical biomarker and therapeutic target.
N-glycan modification and site analysis is essential for understanding glycosylation dynamics and functional significance. Structurally, N-glycans are classified into high-mannose, complex, and hybrid types, with individual glycosylation sites often hosting multiple glycoforms. These site-specific glycoform differences impact protein function, immune response, and therapeutic efficacy, particularly in biopharmaceuticals like monoclonal antibodies and glycoprotein-based biologics. Precise site-specific glycoform characterization is crucial for optimizing protein stability, immunogenicity, and therapeutic performance.
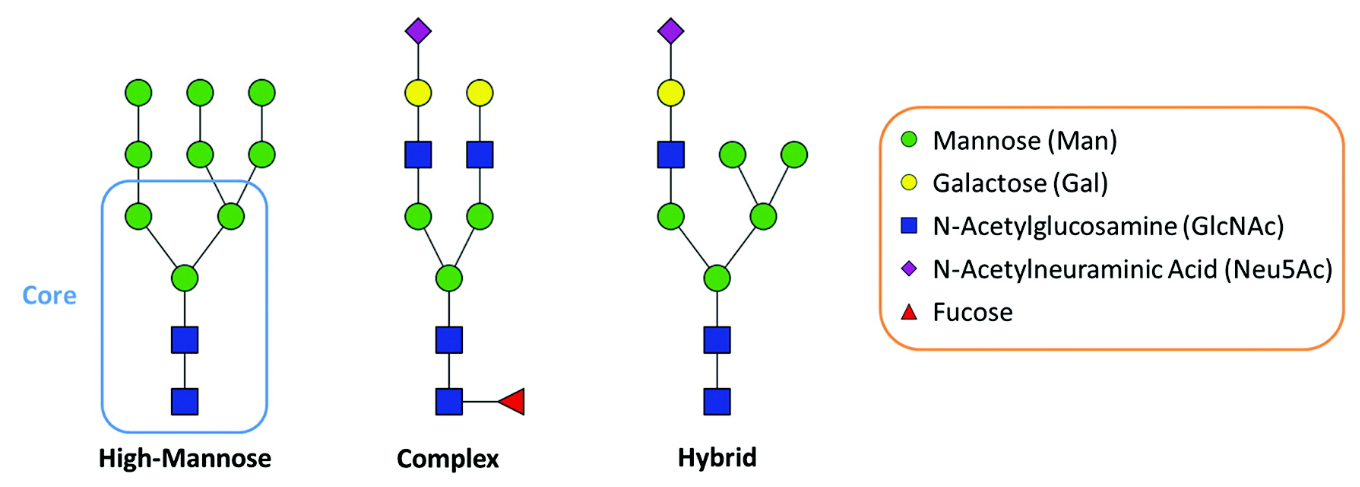
Figure 1. The Three General Types of N-Glycans
Service at MtoZ Biolabs
MtoZ Biolabs provides comprehensive N-Glycan Modification and Site Analysis Services to support biomedical research, biopharmaceutical development, and biomarker discovery. Our platform offers high-resolution, site-specific glycosylation profiling to ensure precise identification, quantification, and structural characterization of N-glycans across diverse biological and therapeutic protein samples. Using cutting-edge LC-MS/MS instruments together with glycopeptide enrichment methods, including HILIC, lectin affinity chromatography, and solid-phase extraction, we deliver confident glycan assignment and detailed glycoform characterization. Advanced fragmentation strategies such as HCD, EThcD, and CID further enhance structural resolution and site-level specificity. To meet the needs of both focused validation and large-scale discovery, MtoZ Biolabs offers two complementary analytical approaches:
1. Target Protein N-Glycan Modification and Site Analysis
For protein-focused investigations, MtoZ Biolabs provides targeted N-glycan analysis to characterize glycosylation sites and associated glycoforms on individual proteins. This approach combines selective glycopeptide enrichment with high-resolution LC-MS/MS to accurately define site occupancy, glycan heterogeneity, and structural features relevant to functional or therapeutic evaluation.
2. Proteome-Wide N-Glycan Modification and Site Analysis
For broader discovery-driven studies, we offer proteome-scale N-glycan analysis to map glycosylation events across complex biological samples. High-throughput LC-MS/MS combined with quantitative glycoproteomics workflows enables comprehensive profiling of glycan structures, abundance changes, pathway involvement, and glycosylation-dependent regulatory networks.
Analysis Workflow
The N-Glycan Modification and Site Analysis workflow begins with protein extraction and purification, followed by enzymatic digestion to release N-glycans. The released glycans undergo separation using chromatography techniques, enabling detailed structural characterization through mass spectrometry. Site-specific glycan profiling is performed to determine glycosylation patterns and microheterogeneity at individual sites. Finally, comprehensive data analysis and interpretation provide insights into glycan composition, distribution, and functional relevance in biological and therapeutic contexts.
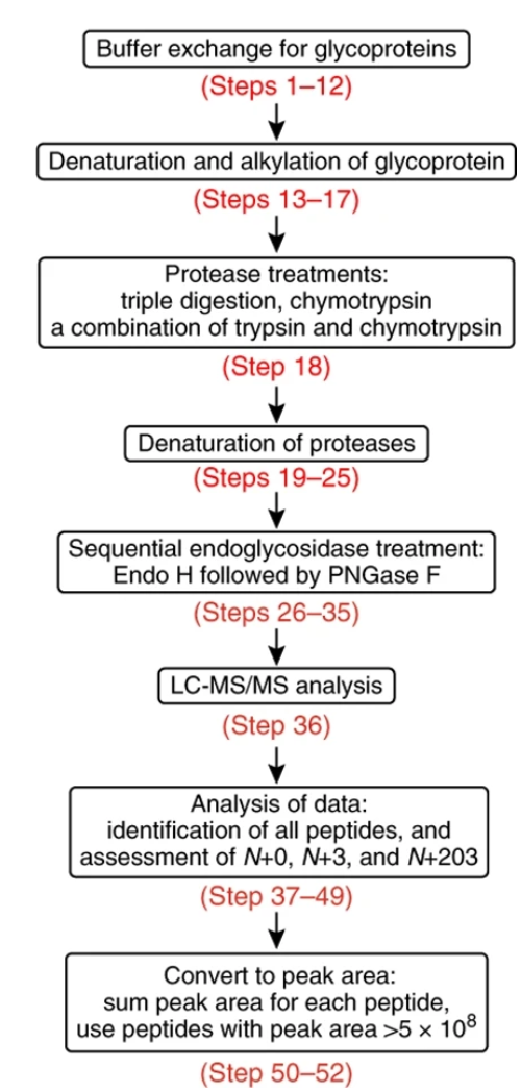
Cao, L. et al. Nat Protoc. 2018.
Figure 2. The Workflow of Site-specific Analysis of Glycoprotein N-glycan Processing
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs established advanced N-Glycan Modification and Site Analysis Services platforms, guaranteeing reliable, fast, and highly accurate analysis service.
2. Customized Analytical Solutions: Tailored workflows for biomarker discovery, disease research, and biologics development, meeting specific project needs.
3. Rapid Turnaround Time: Efficient sample processing and high-throughput analysis accelerate glycan characterization for research and drug development.
Applications
1. Biomarker Discovery
Identifies N-glycan alterations associated with cancer, neurodegenerative disorders, autoimmune diseases, and metabolic syndromes, supporting diagnostic and prognostic biomarker development.
2. Therapeutic Protein Characterization
Ensures batch consistency, stability, and efficacy of monoclonal antibodies, recombinant glycoproteins, and other biopharmaceuticals, meeting regulatory compliance.
3. Cancer Glycomics and Tumor Microenvironment Studies
Investigates glycosylation-mediated immune evasion and metastasis, aiding in cancer biomarker identification and personalized medicine.
4. Immunology and Vaccine Development
Analyzes glycosylation patterns in immune cell receptors and viral glycoproteins, supporting vaccine efficacy studies and immunotherapy research.
5. Glycoengineering and Synthetic Biology
Assists in engineering glycoproteins with optimized glycosylation patterns for enhanced stability, functionality, and therapeutic potential.
Sample Submission Suggestions
Note: Should you have any inquiries regarding sample submission, do not hesitate to reach out. Our expert team is available to assist you at all times.
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on N-Glycan Modification and Site Analysis
4. Mass Spectrometry Images
5. Raw Data
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
N-Glycan Profiling Service | HILIC-UHPLC MS
How to order?







