Negative Staining TEM Analysis Service
- Phosphotungstic acid (PTA): Suitable for most proteins and viral particles; neutral to mildly acidic, with minimal sample disruption.
- Phosphomolybdic acid (PMA): More sensitive to polysaccharides and lipids but may produce heavier background staining.
- Uranyl acetate (UA): Offers high-resolution imaging but is more acidic and can cause protein denaturation or structural compression.
Negative Staining TEM Analysis Service is a morphology-focused analytical solution that combines negative staining sample preparation with transmission electron microscopy (TEM) imaging. It is specifically designed to observe the external structures and spatial distribution of micro- and nanoscale samples such as biological macromolecules, viral particles, and nanomaterials.
In nanostructural biology and materials science research, TEM is widely employed for its high-resolution imaging capabilities, enabling detailed observation of submicron structures such as viruses, protein complexes, liposomes, and nanoparticles. However, many biological specimens are highly hydrated and radiation-sensitive, making direct imaging challenging and often ineffective in producing clear visuals. Negative staining is a simple yet highly effective sample preparation method that significantly enhances image contrast. It has become a critical technique for visualizing ultrastructural features in delicate, low-density specimens.
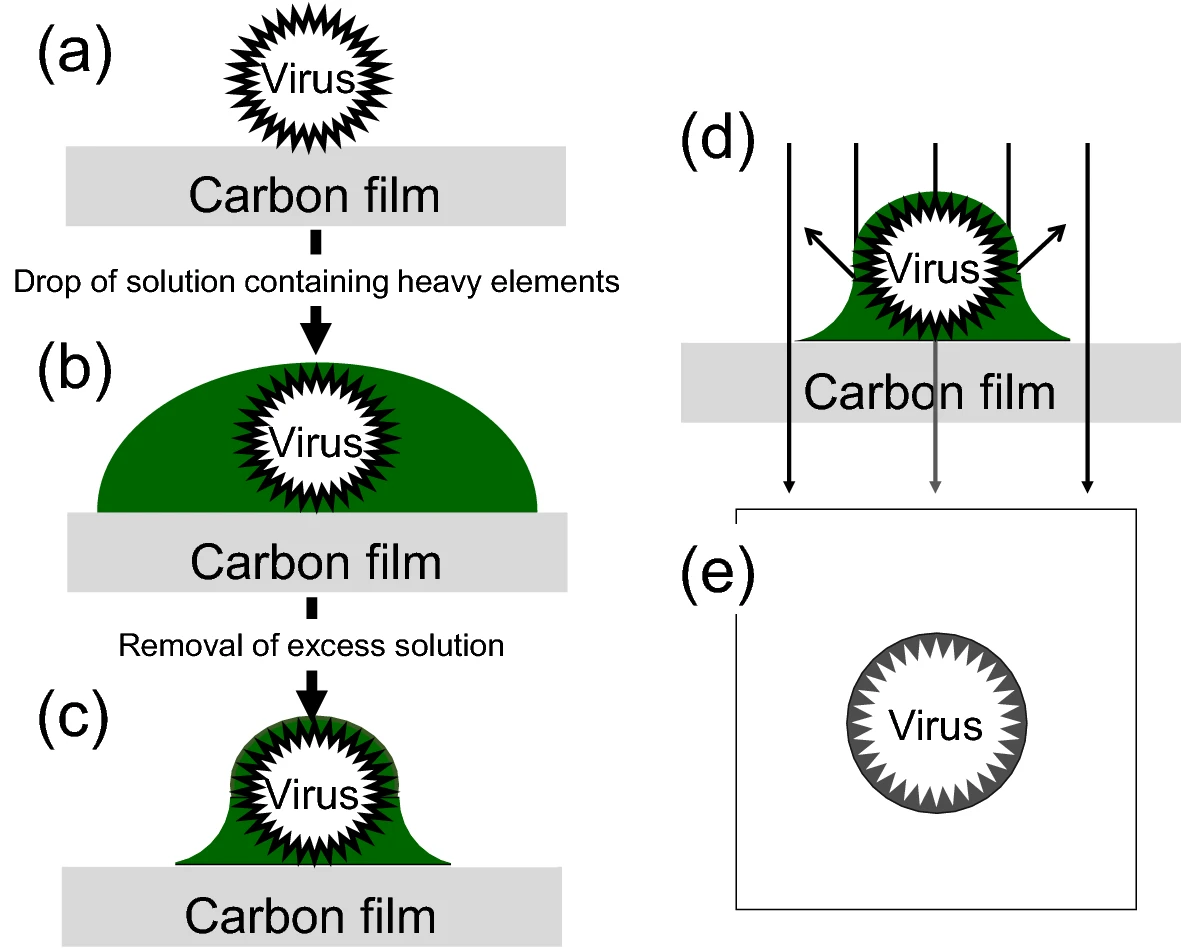
Sahiro K. et al. Scientific reports. 2022.
Negative Staining TEM Analysis refers to the use of TEM following negative staining of the sample. It is a widely applied imaging technique in the study of biological macromolecules and nanoparticles. This method enhances electron density in the background surrounding the sample using heavy metal stains, thereby enabling high-contrast “inverse” imaging of the target particles’ contours. Negative staining is particularly well-suited for visualizing and distinguishing the size, dispersity, and morphological structure of protein complexes, viral particles, liposomes, nanomicelles, and various inorganic nanomaterials.
MtoZ Biolabs offers Negative Staining TEM Analysis Service for morphological observation, particle size evaluation, and structural integrity analysis of nanoscale particles. This service supports visual validation and quality control in virology research, vaccine development, exosome characterization, and nanoparticle-based drug delivery systems.
Analysis Workflow
Negative Staining TEM Analysis Service typically involves the following key steps:
1. Sample Preprocessing
Remove high-salt or protein impurities and adjust the sample to an appropriate concentration to avoid particle aggregation or overlap.
2. Grid Preparation
Use copper grids coated with carbon film. If necessary, perform glow discharge treatment to increase hydrophilicity and promote uniform sample adsorption.
3. Sample Application and Staining
Apply the sample onto the grid and allow it to adsorb. Then add the negative stain (e.g., 2% phosphotungstic acid or uranyl acetate), stain for 30 seconds, and blot dry quickly.
4. Drying and Fixation
Allow the grid to air-dry at room temperature so that the stain embeds the sample outline and creates a high electron density background.
5. TEM Imaging and Data Acquisition
Observe the grid using TEM, capture representative images, and perform particle size analysis or morphology evaluation as needed.
Applications
Negative Staining TEM Analysis Service is applicable to a wide range of research fields, including but not limited to:
Structural Biology
Imaging of overall configurations of viral capsids, protein complexes, ribosomes, nanobodies, etc.
Nanomaterial Characterization
Particle size and distribution analysis of metallic nanoparticles, quantum dots, nanomicelles, polymeric capsules, and more.
Lipid and Vesicle Studies
Morphology and structural integrity assessment of liposomes, exosomes, and vesicle-based drug delivery systems.
Sample Homogeneity Screening
Evaluation of sample purity, structural integrity, and aggregation state.
Cryo-EM Pre-Screening
Preliminary assessment of sample suitability for high-resolution Cryo-EM imaging.
FAQ
Q. How can True Structures be Distinguished from Artifacts Caused by Stain Deposition?
Artifacts in negative-stain TEM images—such as stain crystallization, background precipitates, or carbon film defects—can be mistaken for actual structures. Methods for differentiation include:
1. Morphological regularity: True particles typically exhibit defined size and symmetry, while stain artifacts appear irregular or have fuzzy edges.
2. Reproducibility: Real structures should consistently appear across different fields of view or replicate samples; artifacts are often random.
3. Control comparisons: Use blank grids containing only buffer and stain to identify stain-specific features and rule out interference.
4. Correlative techniques: Use DLS or AFM to verify particle size and confirm the authenticity of structures seen in TEM images.
Q. How does the Choice of Stain Affect Image Quality?
The type of stain directly influences image contrast, background cleanliness, and structural fidelity.
Stain selection should consider sample type, stability, imaging goals, and background noise. Pilot tests using different stains are recommended for optimization.
Case Study
This study utilized Negative Staining TEM to analyze the morphology of novel PEGylated CL4H6 lipid nanoparticles (LNPs). TEM images revealed that the LNPs were spherical with an average diameter of approximately 200 nm, exhibiting uniform shape and size. The particle size measurements were consistent with dynamic light scattering (DLS) data, confirming the structural integrity and dispersibility of the LNPs. These morphological findings support their efficiency in delivering MRTF-B siRNA to human conjunctival fibroblasts.
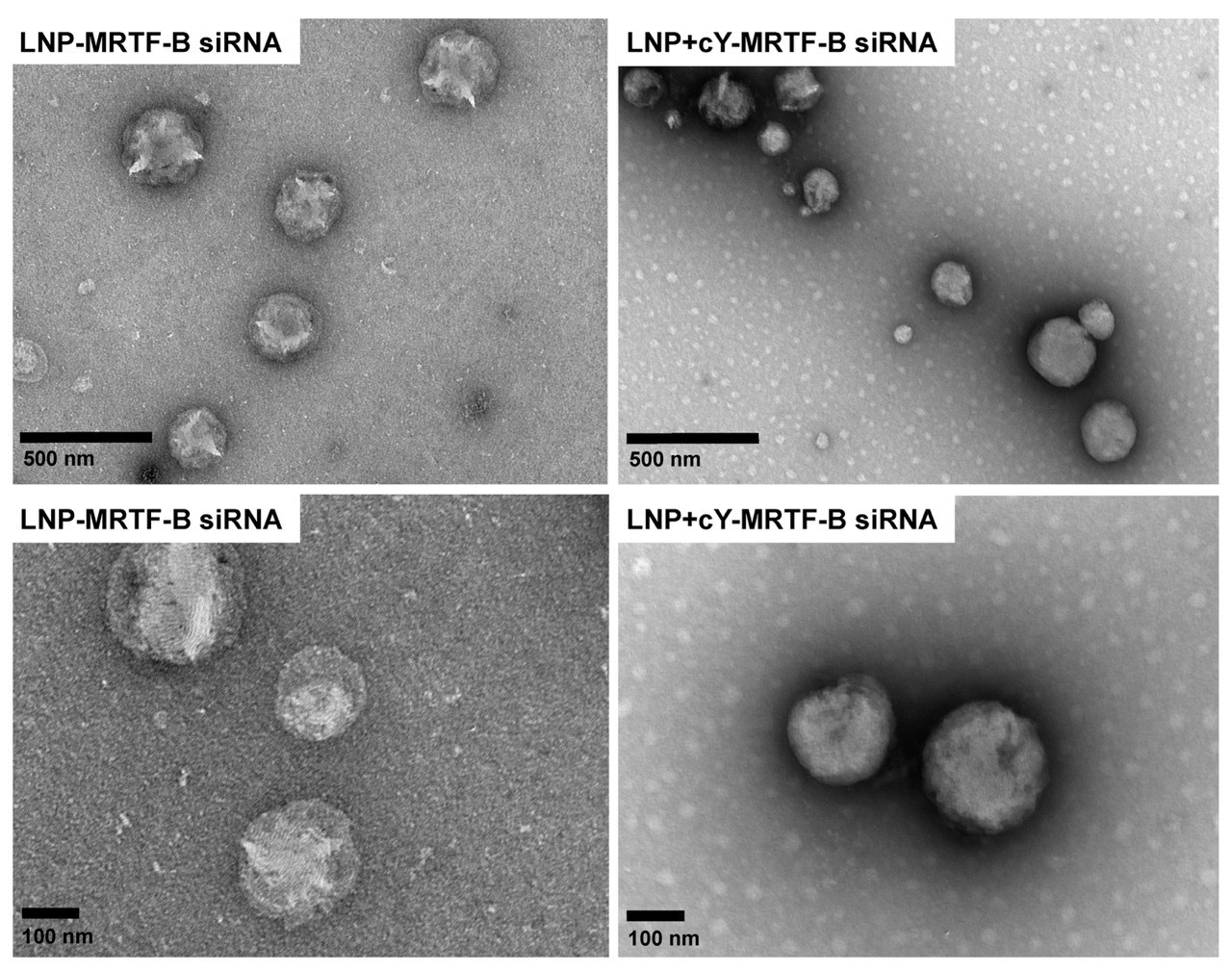
Sanghani A. et al. Pharmaceutics. 2021.
How to order?







