Near UV Circular Dichroism (CD) Spectroscopy Analysis
In structural biology, accurate characterization of the higher-order structure of proteins is essential for understanding their functions and mechanisms. Near-UV Circular Dichroism (CD) spectroscopy, a non-destructive, efficient, and solution-compatible technique, has been widely adopted in studies of protein conformation, drug screening, and quality control of biopharmaceuticals. This review provides a systematic overview of the fundamental principles, practical advantages, and critical relevance of Near-UV CD spectroscopy in protein research.
What Is Near-UV Circular Dichroism (CD) Spectroscopy?
Circular Dichroism (CD) spectroscopy is a spectroscopic method based on the differential absorption of left- and right-circularly polarized light by chiral molecules. The resulting spectrum reflects absorption differences at various wavelengths, typically represented as Δε (difference in molar extinction coefficients). CD spectra are categorized by wavelength range into far-UV (190–250 nm), near-UV (250–320 nm), and visible regions. Near-UV CD spectroscopy focuses on the 250–320 nm range and primarily captures conformational changes in the local environments of aromatic amino acid residues (e.g., tyrosine, tryptophan, phenylalanine) and disulfide bonds. As such, it is particularly well-suited for analyzing protein tertiary structure and conformational stability.
Relationship Between Near-UV CD Spectroscopy and Protein Tertiary Structure
Protein tertiary structure refers to the three-dimensional arrangement formed by non-covalent interactions (e.g., hydrogen bonds, hydrophobic interactions, van der Waals forces) among amino acid side chains. Near-UV CD spectra provide several structural insights:
1. Symmetry of Aromatic Residue Environments
Aromatic side chains exhibit distinct CD spectroscopy responses under different conformations, leading to characteristic spectral features.
2. Sensitive Detection of Conformational Changes
Even subtle environmental perturbations, such as pH shifts, changes in ionic strength, or ligand binding, can produce noticeable alterations in the CD spectroscopy signal.
3. Assessment of Conformational Stability
Protein thermal stability and melting temperature can be evaluated through temperature-dependent CD spectroscopy measurements.
These capabilities make Near-UV CD spectroscopy a vital tool for quality control in the biopharmaceutical industry.
Complementarity with Far-UV CD Spectroscopy
While far-UV CD spectroscopy is extensively used for secondary structure analysis (e.g., determining α-helix and β-sheet content), it provides limited information on tertiary structure. In contrast, near-UV CD yields insights into higher-order structural arrangements and is particularly applicable in cases where detailed conformational evaluation is required.
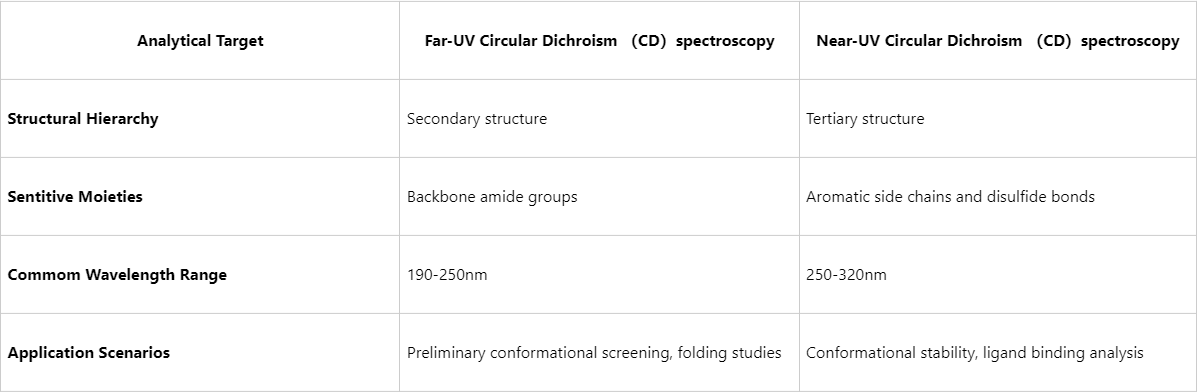
Figure1. Far-UV CD spectroscopy vs Near-UV CD spectroscopy
Therefore, integrating both far- and near-UV CD spectroscopy analyses enables comprehensive monitoring of protein structural hierarchy from secondary to tertiary levels.
Typical Applications
1. Monitoring Protein Conformational Changes
Near-UV CD spectroscopy enables real-time monitoring of minor conformational changes induced by variations in pH, temperature, or ligand interaction. In protein-ligand screening, it aids in identifying whether ligand binding induces conformational rearrangements, thus facilitating the discovery of bioactive candidates.
2. Stability Assessment of Biopharmaceuticals
During the development of monoclonal antibodies and recombinant proteins, stability and batch-to-batch consistency must be rigorously evaluated. Near-UV CD spectroscopy offers a rapid, non-invasive method widely employed in batch consistency verification and optimization of storage conditions.
3. Validation of Drug–Target Interactions
Unlike differential scanning calorimetry (DSC) or surface plasmon resonance (SPR), Near-UV CD spectroscopy can directly assess whether drug binding occurs and whether it induces structural changes in the target protein. This makes it highly valuable for early-stage, high-throughput screening.
Technical Advantages and Limitations
1. Advantages
(1) Non-destructive: Structural insights can be obtained without labeling or chemical modification.
(2) Highly Sensitive: Capable of detecting minor conformational alterations.
(3) User-Friendly: Simple sample preparation and short experimental time make it ideal for routine quality control.
2. Limitations
(1) Requires relatively high protein concentration (typically >0.2 mg/mL).
(2) Incompatible with strongly absorbing buffers (e.g., high-salt or aromatic compound-containing solutions).
(3) Cannot resolve atomic-level structures and often requires integration with complementary techniques such as X-ray crystallography or NMR spectroscopy.
Data Interpretation and Quantitative Analysis
Typical near-UV CD spectra exhibit three characteristic regions:
(1) 255–270 nm: Phenylalanine region
(2) 275–282 nm: Tyrosine region
(3) 285–305 nm: Tryptophan region
By employing spectral deconvolution and curve-fitting approaches, one can quantify the signal contributions and conformational states of individual aromatic residues. This process requires high-quality spectral data and expert interpretation.
As protein structure–function studies gain increasing importance, Near-UV Circular Dichroism (CD) spectroscopy is emerging as a powerful and indispensable technique for conformation analysis. From basic research to drug discovery and biopharmaceutical quality control, it offers irreplaceable structural insights. Leveraging an advanced mass spectrometry platform and robust structural characterization systems, MtoZ Biolabs provides high-quality CD spectroscopy services for protein analysis.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







